Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Parvin Zareian1, Minoo Shaddel2, Mohammad Velayati1*, Hamid Sahamsi1 and Rahim Mohammadi3
Received: August 31, 2017; Published: September 13, 2017
Corresponding author : Mohammad Velayati, Department of Physiology,Faculty of Medicine, AJA University of Medical Sciences, Tehran, Iran
DOI: 10.26717/BJSTR.2017.01.000355
Background: The outgrowth of regenerating axons of a transected peripheral motor nerve is a slow process. The objective was to assess the effect of locally administered gallopamil on transected peripheral nerve regeneration and functional recovery.
Methods: Sixty male healthy white Wistar rats were divided into four experimental groups (n = 15), randomly: In transected group (TC), left sciatic nerve was transected and stumps were fixed in the adjacent muscle. In treatment group defect was bridged using silicone tube (SIL/ Gallopamil) filled with 10 μL gallopamil (100 ng/mL). In silicone conduit group (SIL), the tube was filled with phosphate-buffered saline alone. In sham-operated group (SHAM), sciatic nerve was exposed and manipulated. Each group was subdivided into three subgroups of five animals each and regenerated nerve fibers were studied 4, 8 and 12 weeks after surgery.
Results: Behavioral testing, biomechanical studies, sciatic nerve functional study, gastrocnemius muscle mass and morphometric indices confirmed faster recovery of regenerated axons in SIL/Gallopamil than SIL group (p < 0.05). In immunohistochemistry, location of reactions to S-100 in SIL/Gallopamil was clearly more positive than that in SIL group.
Conclusion: When loaded in a silicone tube gallopamil accelerated and improved functional recovery and morphometric indices of sciatic nerve. This may have clinical implications for the surgical management of patients after nerve transaction.
Keywords: Peripheral Nerve Repair, Sciatic, Gallopamil, Local
Abbreviations: TC: Transected Group; SIL: Silicone Conduit Group; SHAM: Sham Operation Group; BBB: Basso, Beattie and Bresnahan; SFI: Sciatic Functional Index; N-CAM: Nerve Cell Adhesion Molecule
The outgrowth of regenerating axons of a transected peripheral motor nerve is a slow process. After an initial delay, which is necessary for the cell bodies to compensate for the retrograde effects of axonal transection, the regenerating axonal sprouts cross the site of injury, reach the distal stump, and grow down the nerve to their peripheral terminations [1]. The conduits act to guide axons sprouting from the regenerating nerve end, provide a microenvironment for diffusion of neurotrophic and neurotropic factors secreted by the injured nerve stump, as well as help protect from infiltration of fibrous tissue [2]. It has been reported that using silicone tubes in bridging of nerve defects could be promising because it is inert and does not induce extensive scarring or degeneration after implantation [3]. The advantages like no donor morbidity, availability, affordability and no foreign reactions make silicone rubber chamber an attractive alternative to other standard grafts [4,5].
Calcium channel blockers have been used to reduce the morbidity and mortality associated with delayed ischemic deficits in patients with subarachnoid hemorrhage. In addition to this activity in the central nervous system, they have been shown promise in multiple rodent models as a possible pharmacologic treatment for facial nerve injury [6-10]. To the best knowledge of the authors, the literature is poor regarding the beneficial local effects of gallopamil (a calcium channel blocker) on transected sciatic nerve. Aimed to study local effects of gallopamil on sciatic nerve regeneration, a study was designed to determine if local gallopamil could in fact reduce dysfunction after nerve injury in the rat sciatic nerve transection model. Assessment of the nerve regeneration was based on behavioral, biomechanical, functional, histomorphometric and immuohistochemical (Schwann cell detection by S-100 expression) criteria 4, 8 and 12 weeks after surgery.
Sixty male Wistar rats weighing approximately 250g were divided into four experimental groups (n = 15), randomly: shamoperation group as normal control (SHAM), transected control (TC), silicone conduit (SIL) and Gallopamil treated group (SIL/ Gallopamil). Each group was further subdivided into three subgroups of five animals each and studied 4, 8 and 16 weeks after surgery. Two weeks before and during the experiments, the animals were housed in individual plastic cages with an ambient temperature of (23±3) °C, stable air humidity and a natural day/ night cycle. The rats had free access to standard rodent laboratory food and tap water. All measurements were made by two blinded observers unaware of the analyzed groups.
Animals were anesthetized by intraperitoneal administration of ketamine-xylazine (ketamine 5%, 90mg/kg and xylazine 2%, 5mg/kg). The procedure was carried out based on the guidelines of the Ethics Committee of the International Association for the Study of Pain [11]. The University Research Council approved all experiments. Following surgical preparation in the sham-operation group, the left sciatic nerve was exposed through a gluteal muscle incision and after careful homeostasis the muscle was sutured with resorbable 4/0 sutures, and the skin with 3/0 nylon. In TC group, the left sciatic nerve was transected proximal to the tibio-peroneal bifurcation where a 7mm segment was excised, leaving a 10mm gap due to retraction of nerve ends. Proximal and distal stumps were fixed in the adjacent muscle with 10/0 nylon epineurial suture. No graft was interposed between the stumps. In the SIL group, a 7 mm nerve segment was resected to produce a 10 mm nerve gap after retraction of the nerve transected ends. The gap was bridged using a silicone conduit, entubulating 2 mm of the nerve stump at each end. The silicone conduit was 2 mm in diameter with 2 thicknesses in wall. Two 10/0 nylon sutures were used to anchor the conduit to the epineurium at each end. In gallopamil treated group (SIL/Gallopamil) the conduit was filled with 10 μl Gallopamil (100 ng/mL). The animals were anesthetized and euthanized with transcardiac perfusion of a fixative containing 2% paraformaldehyde and 1% glutaraldehyde buffer (pH 7.4) 4, 8 and 12 weeks after surgery.
Functional recovery of the nerve was assessed using the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale for rat hind limb motor function [12]. Although BBB is widely used to assess functional recovery in spinal cord injured animals, however, it has been demonstrated that it could be most useful in assessment of never repair processes in peripheral nerve injuries [13]. Scores of 0 and 21 were given when there were no spontaneous movement and normal movement, respectively. A score of 14 shows full weight support and complete limbs coordination (Table 1). BBB recordings were performed by a trained observer who was blinded to the experimental design. The testing was performed in a serene environment. The animals were observed and assessed within a course of a 4 minute exposure to an open area of a mental circular enclosure. BBB scores were recorded once before surgery in order to establish a baseline control and again weekly thereafter to assess functional recovery during 12 weeks.
Table 1: Biomechanical analyses of regenerated nerves for each of the experimental groups: values are given as mean ± SD.
*: The mean difference is significant at the 0.05 level vs. SIL group.
Sciatic Functional Index
Walking track analysis was performed 4, 8 12 and 16 weeks after surgery based on the method of others [14]. The lengths of the third toe to its heel (PL), the first to the fifth toe (TS), and the second toe to the fourth toe (IT) were measured on the experimental side (E) and the contralateral normal side (N) in each rat. The sciatic function index (SFI) of each animal was calculated by the following formula:
In general, SFI oscillates around 0 for normal nerve function, whereas around -100 SFI represents total dysfunction. SFI was assessed in the NC group and the normal level was considered as 0. SFI was a negative value and a higher SFI meant the better function of the sciatic nerve.
Following electrophysiological assessments, the regenerated nerves were harvested and placed in a normal saline bath at room temperature. The samples were then fixed between frozen fixtures in a mechanical apparatus. The TA.XT Plus Texture Analyzer mechanical test device was used for the assessment (Stable Micro Systems, Surrey GU7 1YL, UK). After 5 minutes, the frozen fixtures were tightened to ensure that no slippage occurred during testing. The initial length was set to 10 mm. Each sample was stretched at a constant rate of 1 mm/min. The load and displacement were sampled 5 times per second. Each sample was stretched to complete tensile failure. Samples were kept wet moist during testing using a drop of normal saline solution to the nerve segments.
Nerve mid-substance in SIL group, nerve mid-substance in gallopamil treated group, midpoint of normal sciatic nerve (SHAM) and regenerated mid substance of TC group were harvested and fixed with glutaraldehyde 2.5%. They were post fixed in OsO4 (2%, 2h), dehydrated through an ethanol series and embedded in Epon. The nerves were cut in 5 μm in the middle, stained with toluidine blue and examined under light microscopy. Morphometric analysis was carried out using an image analyzing software (Image-Pro Express, version 6.0.0.319, Media Cybernetics, Silver Springs, MD, USA). Equal opportunity, systematic random sampling and twodimensional dissector rules were followed in order to cope with sampling-related, fiber location related and fiber size related biases [15].
Recovery assessment was also indexed using the weight ratio of the gastrocnemius muscles 12 weeks after surgery. Immediately after sacrificing of animals, gastrocnemius muscles were dissected and harvested carefully from intact and injured sides and weighed while still wet, using an electronic balance.
In this study, anti-S-100 (1:200, DAKO, USA) was used as marker for myelin sheath. Specimens were post fixed with 4% paraformaldehyde for 2h and embedded in paraffin. Prior to immunohistochemistry nerve sections were dewaxed and rehydrated in PBS (pH 7.4). Then the nerve sections were incubated with 0.6% hydrogen peroxide for 30 minutes. To block non-specific immunoreactions, the sections were incubated with normal swine serum (1:50, DAKO, USA). Sections were then incubated in S-100 protein antibody solution for 1h at room temperature. They were washed three times with PBS and incubated in biotynilated antimouse rabbit IgG solution for 1h. Horseradish peroxidase-labelled secondary antibody was applied for 1h. After that all sections were incubated with 3, 3’- diaminobenzidine tetrahydrochloride chromogene substrate solution (DAB, DAKO, USA) for 10 min. The results of immunohistochemistry were examined under a light microscope.
The results were expressed as means ± SD. Statistical analyses were performed using PASW 18.0 (SPSS Inc., Chicago, IL, USA). Model assumptions were evaluated by examining the residual plot. Results were analyzed using a factorial ANOVA with two between subject’s factors. Bonferroni test for pairwise comparisons was used to examine the effect of time and treatments. The differences were set at P < 0.05.
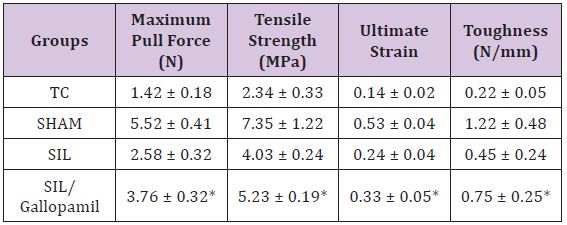
BBB recovery: In order to assess hind limb recovery, the open field locomotor was used. BBB scores compared to the baseline (Figure 1). All experimental groups, except for sham, showed the greatest degree of functional deficit one week after surgery. The gallopamil treated group showed significant improvement in locomotion of the operated limb compared to the SIL group during the study period (P < 0.05).
Figure 1: BBB score for all experimental groups. Local administration of Gallopamil with silicon conducting gave better scores than in SIL group. Data are presented as mean ± SD. * P < 0.05 vs. SIL group.
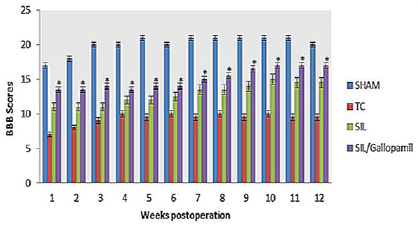
SFI Outcome: Sciatic Function Index (SFI) values in all four experimental groups are being shown (Figure 2). Prior to surgery, SFI values in all groups were near zero. After the nerve transection, the mean SFI decreased to -100 due to the complete loss of sciatic nerve function in all animals. The statistical analyses revealed that the recovery of nerve function was significantly (P < 0.05) different between SIL/Gallopamil and SIL groups and application of the gallopamil in silicon conduit significantly accelerated functional recovery in the course of time.
Figure 2: Box-and-whisker plots of sciatic nerve function index values (SFI) in each experimental group during the study period. Local administration of Gallopamil gave better results in functional recovery of the sciatic nerve than in SIL group.
Biomechanical Measurements: Maximum pull force (Fmax) of normal sciatic nerve was found to be 5.50 ± 0.40. Fmax of nerve samples in experimental groups (Table 1). Fmax in SIL/Gallopamil was significantly higher than that in SIL group (P < 0.05). Tensile strength, the amount of force per unit of initial cross-sectional area at tensile failure, was measured based on Fmax and nerve cross sectional area. 12th week assessment revealed tensile strength of regenerated nerves treated with gallopamil was higher than those in SIL group (P < 0.05). Ultimate strain, the amount of elongation divided by the initial specimen length achieved at the point of tensile failure, in SIL/Gallopamil group was significantly higher than that in SIL group (P < 0.05). Toughness, reflecting the properties of antideformation and anti-fracture of nerve, was determined by the nerve itself and could reflect “looseness” or “toughness” of nerve. Toughness in SIL/Gallopamil group was significantly higher than that in SIL group (P < 0.05).
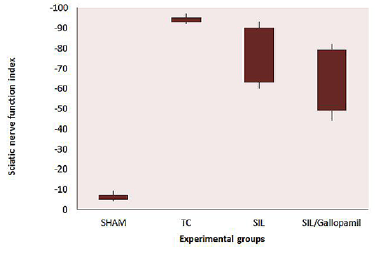
Histological and Morph metric Findings: The Gallopamil treated group presented significantly greater nerve fiber, axon diameter, and myelin sheath thickness during study period, compared to SIL animals (P < 0.05). Sham-operation group presented significantly greater nerve fiber and axon diameter, and myelin sheath thickness compared to SIL/Gallopamil and SIL groups animals (Figures 3a & 3c). In case of myelin thickness there was no significant difference between SIL/Gallopamil and SIL groups, morph metrically (P >0.05).
Muscle Mass Measurement: The mean ratios of gastrocnemius muscle weight were measured at the end of the study period. There was a statistically significant difference between the muscle weight ratios of the SIL/Gallopamil and SIL groups (P < 0.05). The results showed that in the gallopamil treated group, the muscle weight ratio was larger than in the SIL group, and weight loss in the gastrocnemius muscle was ameliorated by Gallopamil local administration (Figure 3d).
Figure 3: a. Line graph shows the quantitative results of fiber counting. Both groups of SIL and SIL/Gallopamil showed the lower number of fibers than the Sham-operated group even at the end of the study. b. Line graph shows the quantitative results of mean diameter of nerves fibers. Both groups of SIL and SIL/Gallopamil showed the lower mean diameter of nerve fibers than the Sham-operated group even at the end of the study. c. Line graph shows the quantitative results of myelin thickness. Both groups of SIL and SIL/Gallopamil showed the lower mean myelin thickness than the Sham-operated group even at the end of the study. d. Measurement of gastronomius muscle. The gastronomius muscle of both sides (injured left and intact right) was removed and weighted in experimental groups 12 weeks after surgery. Data are presented as mean ± SD. * P < 0.05 vs SIL group.
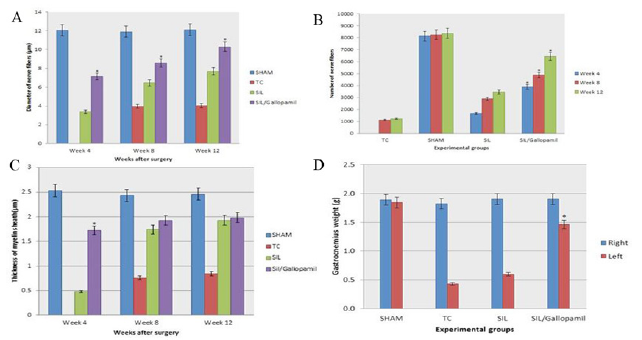
Figure 4: Immunohisto chemical analysis of the regenerated nerves 12 weeks after surgery from middle cable (A) SHAM, (B) SIL, (C) and (D) SIL/Gallopamil. There is clearly more positive staining of the myelin sheath-associated protein S-100 (arrow) within the periphery of nerve, indicating well organized structural nerve reconstruction in Gallopamil treated nerve. Scale bar: 10 μm.

Immunohistochemistry: Immunoreactivity to S-100 protein was extensively observed in the cross sections of regenerated nerve segments. The expression of S-100 protein signal was located mainly in the myelin sheath. The axon also showed a weak expression indicating that Schwann cell-like phenotype existed around the militated axons (Figure 4). In both SIL/Gallopamil and SIL groups, the expression of S-100 and the findings resembled those of the histological evaluations.
The results of the present study showed that administration of gallopamil into a silicon conduit resulted in faster functional recovery of the sciatic nerve during the study period. Left gastrocnemius muscle weight was significantly greater in the SIL/Gallopamil group than in the SIL group, indicating indirect evidence of successful end organ reinnervation in the gallopamil treated animals. At week 12 quantitative morphometrical indices of regenerated nerve fibers showed significant differences between the SIL and SIL/Gallopamil groups, indicating a beneficial effect of topical application of gallopamil on the nerve regeneration. Although both morphological and functional data have been used to assess neural regeneration after induced crush injuries, the correlation between these two types of assessment is usually poor [16]. Classical and newly developed methods of assessing nerve recovery, including histomorphometry, retrograde transport of horseradish peroxidase and retrograde fluorescent labeling do not necessarily predict the reestablishment of motor and sensory functions [17].
Although such techniques are useful in studying the nerve regeneration process, they generally fail in assessing functional recovery [16]. Therefore, research on peripheral nerve injury needs to combine both functional and morphological assessment. Castaneda et al. [18] suggested that arrival of sprouts from the proximal stump at the distal nerve stump does not necessarily imply recovery of nerve function. Information taken from BBB scale may be invaluable in evaluation of peripheral nerve process. Results of the present study showed that the gallopamil treated animals had been improved in locomotion of the operated limb compared to the SIL group during the study period. Walking track analysis has frequently been used to reliably determine functional recovery following nerve repair in rat models [14].
A wide variety of materials have been used to produce nerve guides, including non- biodegradable and biodegradable materials. Because of its inert and elastic properties, the silicon tube was one of the first and most frequently used to bridge the transected nerves [18]. Membranes of the proximal and distal portions of the transected axons are resealed 5-30 min after the transection [19]. Thereafter, the proximal stump gradually regrows, fostered by the neural cell surface molecule of the transmembrane glycoprotein Ll, nerve cell adhesion molecule (N-CAM), myelinassociated glycoprotein PO, and the extracellular matrix components laminin and tenascin [20]. The regenerating axons are guided by the Schwann cell processes and their growth within the Schwann cell basal lamina tubes is synchronous with the withdrawal and degeneration of the axonal remnants of the distal stump [1,20].
Calcium ions play a crucial role in depolarization, outgrowth, excitability, aging, learning, and cell proliferation in short, neuronal plasticity [21]. It is well known that peripheral nerve injury disrupts the permeability barrier function of the plasma membrane, allowing an influx of Ca2+ down a steep electrochemical gradient between the outside and the inside of the cell [22]. The resultant intracellular free Ca2+ overload triggers a wide array of chain reactions, which eventually may lead to cell death [23]. Therefore, an agent preventing the excessive influx of Ca+ might attenuate cellular damage caused by mechanical neuronal injury and thus improve neuronal recovery [24]. By reducing the amount of calcium influx into the axoplasm of the resprouting nerve fiber, the treatment with gallopamil may provide the necessary optimum level (“set-point”) of Ca+ influx that promotes accelerated growth cone elongation [25]. However, it further reduces the buffering capacity of the terminals for Ca+, which might render their responsiveness to Ca2+ even stronger [26,27].
Even though our preliminary study shows the neuroprotective action of local gallopamil in peripheral nerve injuries, determining the molecular mechanisms leading to the neuroprotective action remains needs to be investigated. We have not given the histological and molecular evidence for neuro protective action of Gallopamil. This may be considered as a limitation to our study. Therefore, the authors stress that the aim of the current investigation was to evaluate a single local dose and clinical treatment potential of Gallopamil on transected sciatic nerve regeneration including functional assessments of the nerve repair, a case not considered in previous studies. The results of the present study indicated that a single local administration of Gallopamil at the site of transected nerve could be of benefit after silicon conduit tubulization. Detailed mechanism of neuroprotective action remains to be investigated.
The present study demonstrated that a single local application of gallopamil could accelerate functional recovery after transection of sciatic nerve and may have clinical implications for the surgical management of patients after facial nerve transection. Thus, dose response studies should be conducted for Gallopamil to determine the combination of the graft and the compound that achieve maximal efficacy in nerve transaction models.


