Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Mohamed Ziaulla*
Received: October 02, 2017; Published: October 13, 2017
Corresponding author: Mohamed ziaulla, Department Of Chemistry, Impact College of Engineering and Applied Sciences, Bangalore- 92, India
DOI: 10.26717/BJSTR.2017.01.000437
Keywords : 2-(4-(methylthio) phenyl)-1H-benzo[d]imidazole (1) derivative, C-H…N, N-H…N and Methylsulfanylphenyl)-1Hbenzimidazol-3- ium bromide (2) N-H…Br hydrogen bonds involving the bromide ion. Moreover, C-H…S interactions result in chains of molecules along the c axis.
Benzimidazoles and their derivatives exhibit a number of important pharmacological properties, such as antihistaminic [1], anti-ulcerative [2], anti allergic [3], and antipyretic [4]. In addition, benzimidazole derivatives are effective against the human cytomegalovirus (HCMV) [5] and are also efficient selective neuropeptide Y Y1 receptor antagonists [6]. We report here in the Crystal structure of 2-(4-(methylthio) phenyl)-1H-benzo[d] imidazole (1) and 2-(4-Methylsulfanylphenyl)-1Hbenzimidazol-3- ium bromide (2).
The X-ray diffraction data for the compound 1 was collected on a Bruker Smart CCD Area Detector System, using MoKα (0.71073Å) radiation for the crystal. Intensity data were collected up to a maximum of 26.37° in the w–ф scan mode. The data were reduced using SAINTPLUS [7]. The structure was solved by direct methods using SHELXS97 [8] and difference Fourier synthesis using SHELXL97 [8]. The positions and anisotropic displacement parameters of all non-hydrogen atoms were included in the fullmatrix least-square refinement using SHELXL97 [8] and the procedures were carried out for a few cycles until convergence was reached. A total of 17827 reflections were collected, resulting in 2520 [R(int) = 0.0847] independent reflections of which the number of reflections satisfying I>2 σ(I) criteria was 1382. The R factor for observed data finally converged to R= 0.0736 with wR2 = 0.1351 in the compound. Molecular diagrams were generated using ORTEP [9]. The mean plane calculation was done using the program PARST [10] (Figures 1 & 2).
Figure 1 : Molecular structure of the title compound with the atomic-numbering scheme. Dotted line indicates intramolecular C4-H4…N2 interaction.
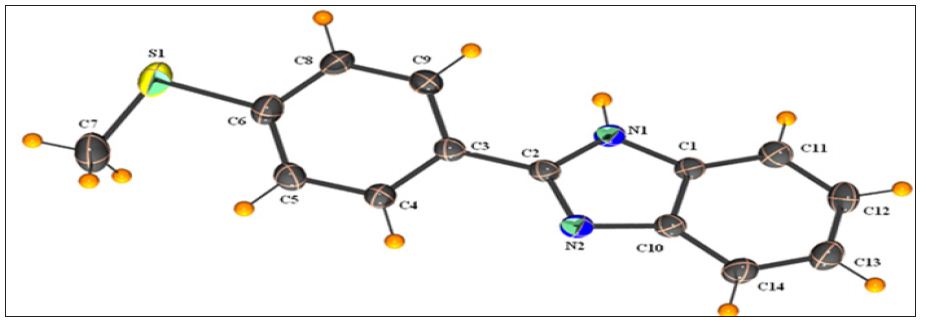
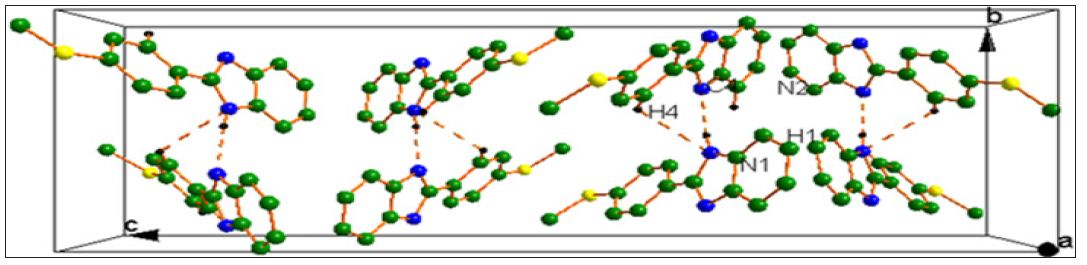
Figure 2 : Packing diagram viewed along the ‘a’ axis. Dotted lines indicates C4-H4…N1 and N1-H1….N2 intermolecular interactions.
The X-ray diffraction data for the compound 2 was collected on a Bruker Smart CCD Area Detector System .In the compound, there is one benzimidazole thiomethyl phenyl cation and one Branion in the asymmetric unit. The expected proton transfer from HBr to benzimidazole thiomethyl phenyl occurs at atom N1 of the benzimidazole ring. Consequently, atom N1 shows quaternary character and bears a positive charge. In the molecule, the benzimidazole and thiomethyl phenyl rings are planar inclined at an dihedral angle 2.133(2)° between them. The molecular structure is primarily stabilized by strong intramolecular N-H…Br hydrogen bond Further, the crystal structure is stabilized by intermolecular interactions into three dimensional framework structure by the combination of C-H…S and N-H…Br. The C-H…S and N-H…Br interactions together generates tetramers linking the molecules into chain like pattern along crystallographic ‘a’ axis. (Figures 3 & 4)
Figure 3 : ORTEP view of the title compound with the atomic-numbering scheme.

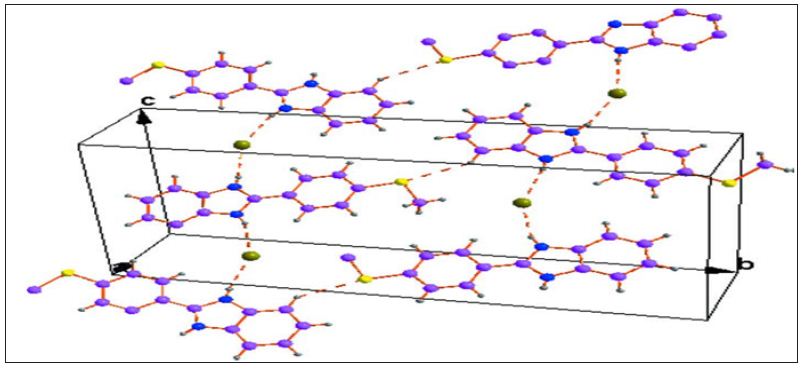
Figure 4 : A unit cell packing of the title compound showing Intermolecular interactions with dotted lines.
ZIA is thankful to Dr. Paul Mathulla, Chairman of governing council and Dr. Alice Abraham, President, Impact Group of Institutions for their constant support and encouragement.


