Abstract
Lignin has shown to have a great potential in replacing oil in many applications, including resins, polyols, polymers, coatings, and composites. The current case study describes the application of the selected lignin oligomeric fraction produced by a novel enzymatic lignin fractionation process (METNIN™) as a suitable lignopolyol to completely replace a commercial polyol in polyurethane rigid foam formulations. More specifically, the preparation of liquid lignopolyols with a high lignin content (up to 40%) by oxypropylation and its full utilization in the polyurethane rigid foam formulation was successfully demonstrated. In addition, selected technical specifications of foam demonstrators were characterized, including closed cell count, water uptake and compression characteristics. These specifications were all within industrial standards for rigid foam applications. The lignin loading in the lignopolyol was a major factor determining the properties of the foam and further studies are needed to further optimised and fully validate the specifications.
Keywords: Enzyme; Oxidation; Lignin; Depolymerisation; Polyol; Polyurethane foam
Introduction
Lignin valorization to added-value products has been a longstanding
challenge of biorefineries. Most commonly the lignin side
stream from biofuel and biorefinery plants is simply dried and burnt
as bio-energy carrier in incineration facilities or co-generation
units to generate useful secondary energy (e.g. heat and electricity).
However, lignin valorization is mandatory for the commercially
feasible conversion of plant biomass into industrial and consumer
goods [1]. Lignin has shown to have great potential in replacing oil
in many applications, including resins, polyols, polymers, coatings,
and composites [2]. The key to success is to refine the complex
molecules to have specific chemical characteristics that match
the end-user application need. Recently, the integration of a lignin
polymer as an inactive filler or an active polyol component in
Polyurethane (PUR) foam formulations have gained great interest,
both in academic and industrial sectors [3,4]. Polyurethane
products can be found in building and construction, transportation,
furniture and bedding, appliances, packaging, textiles, fibers &
apparel, machinery & foundry, electronics, footwear. In addition,
polyurethanes are commonly used in several medical applications
including catheter and general-purpose tubing, hospital bedding,
surgical drapes, wound dressings, as well as in a variety of injection
molded devices. The current polyols used in PUR manufacture are of
fossil origin and do not possess flame retardant properties, leading
to the addition of synthetic retardants (typically halogenated
organo-phosphates) to PUR mixtures with health concerns. The
lignin-based polyol (lignopolyol) bioequivalent possesses intrinsic
flame-retardant properties due to its phenol-based structure,
potential serving as a drop-in replacement of fossil polyols in PUR
foam manufacturing. However, to this date, rather poor reactivity
and heterogeneous and complex structure of lignin polymer have
restricted its wide-spread application in PUR applications.
Despite recent advances in catalytic processes for the
valorization of lignin into materials and chemicals, still no
established technology exists for its efficient depolymerization and reactivity enhancement [5,6]. This is due to harsh conditions for
lignin isolation together with the prevalence of highly recalcitrant
intra-molecular lignin linkages, and repolymerization tendency
of phenoxy radicals. Enzymatic depolymerization of lignin is
envisaged as one of the potential breakthrough applications for
lignin valorization [7]. However, matching the optimal operation
conditions of the enzyme with the optimal processibility of lignin
has been a challenge. Recently, alkaliphilic laccase enabling the
enzymatic operations at the conditions to better match the optimal
processibility of crude lignin, i.e. alkaline aqueous conditions (pH
10-11) has been demonstrated [8]. In addition, a novel lignin
valorization technology utilizing alkaliphilic laccases for the
enzymatic oxidation of lignin in alkaline aqueous conditions (pH
10-11) combined with cascading membrane operations has been
recently introduced by MetGen Oy [9].
This technology, called METNIN™, has shown to able to produce different molecular size lignin fractions (from oligomeric down to a mixture of tri-, di and monomeric units) with a distinct molecular weight distribution and a low polydispersity together with more favourable physicochemical properties. METNIN™ process enables the utilization and potentially better applicability of lignin in a variety of application areas, including polyurethane foams. The current case study describes the application of a selected lignin oligomeric fraction produced by METNIN™ process as a suitable lignopolyol to completely replace a commercial polyol in PUR foam formulation. More specifically, the preparation of liquid lignopolyols by oxypropylation of METNIN™ oligomeric lignin fraction and its full utilization in the polyurethane rigid foam formulation is demonstrated. In addition, selected technical specifications of PUR demonstrators are characterized.
Material and Methods
Lignin Characteristics
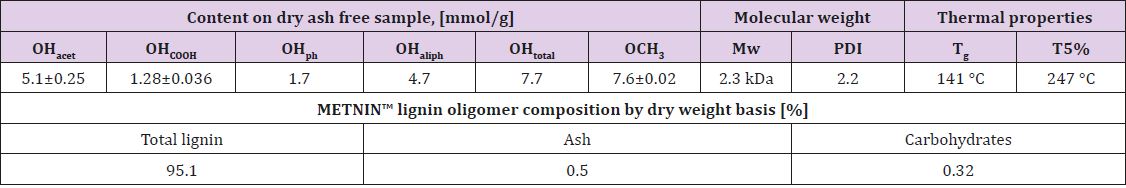
A crude lignin extracted from a woody (birch) biomass was supplied by SWEETWOODS project [10]. The selected lignin oligomeric fraction was obtained by the fractionation of the crude lignin by METNIN™ enzymatic lignin valorization process (MetGen Oy, Finland) [9]. METNIN™ process produces an aqueous alkaline solution of lignin fractions (pH 10-11). For this study, METNIN™ lignin fraction was extracted as a dry powder using acid precipitation protocol adapted from Sameni et al. [11]. An example of the sample is shown in Figure 1A. Selected physicochemical properties of METNIN ™ lignin oligomer fraction after extraction are collected in Table 1. METNIN™ lignin oligomer shows high purity (>95%) with low ash and carbohydrate content. Average molecular weight (Mw) and polydispersity index (PDI) were determined by HPLC chromatographer 1200 CompactLC with UV detector (Agilent Technologies), equipped with size exclusion column set MCX 1000 Å + 100 000 Å 10 μm, 8 x 300 mm and with pre-column MCX 10 μm, 8 x 50 mm (Polymer Standards Service). Isocratic mode with 0.1 M NaOH eluent flow 0.6 mL/min at RT was used with run time of 45 min.
Figure 1: LEFT: METNIN™ lignin oligomer (as a dry powder) after acid precipitation. MIDDLE: Lignopolyol liquid after oxypropylation reaction (Formulation A). RIGHT: Polyurethane foam blocks with Formulation A, Formulation B and Reference (with commercial polyols).
Table 1: Selected physicochemical characteristics and composition of METNIN™ lignin oligomer. Values for acetylated hydroxyl group (OHacet), carboxylic group (OHCOOH), phenolic group (OHph), aliphatic OH (OHaliph), methoxy (OCH3) content, average molecular weight (Mw), polydispersity index (PDI), glass transition temperature (Tg) and temperature at which 5% of weight loss is observed (T5%) are presented- .
Lignin samples were monitored at 358 nm. Molecular mass
standards (polystyrene sulfonate sodium salt standards Mp = ~0.9
to 976 kDa, Polymer Standards Service) were monitored at 254
nm. Lignin model compounds with MW between 180 and 638 Da
monitored at 358 nm were used to extend the standard curve. Data
was acquired with EzChrom Elite Compact software. Ash content
was determined by EN 14775:2009 standard. Total lignin content
is the sum of Klason (acid insoluble) lignin content determined by
the gravimetric method according to TAPPI T222 standard and acid
soluble lignin content determined by the UV spectrophotometric
method according to TAPPI 250 UM standard. Carbohydrate (i.e.
monomeric sugars content after carbohydrate complex hydrolysis)
was determined by a gas chromatographic analysis with modified
alditol acetate method [12]. Acetylated OH group (OHacet) content
was determined by the modified Verley and Bolsing method [13].
Methoxy (OCH3) content was determined by Zeisel-Viebock-
Schwapapch method [13]. Total hydroxylic group (OHtotal) content
was determined by the wet chemistry method (acetylation by
acetic anhydride followed by free acid potentiometric titration by
0.1 M NaOH) [13].
Carboxylic group (OHCOOH) content was determined by wet
chemistry chemisorption method using calcium acetate. The total
acidic groups (phenolic + carboxylic) content was determined by the
acid-base back conductometric titration method [13]. The content
of phenolic groups (OHph) was calculated from the difference
between the values of conductometric and chemisorption methods.
The aliphatic OH (OHaliph) content was calculated by the following
relation: OHaliph = OHacet +OHCOOH- OHph. analysis (TGA) to determine
the temperature at which 5% of weight loss is observed (T5%)
was conducted in following conditions: sample weight 8.0-8.5
mg, heating rate 10˚C/min, atmosphere nitrogen. The differential
scanning calorimetry (DSC) used to determine the glass transition
temperature (Tg) was performed in the temperature range of
0-180˚C with a heating rate of 10 ˚C/min.
Lignopolyl Preparation and Characterisation
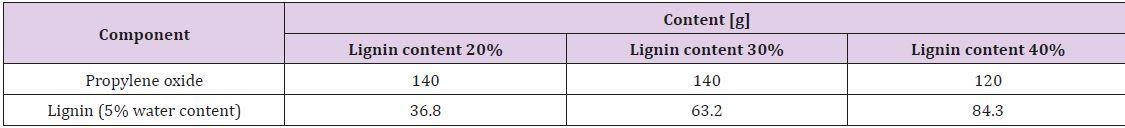
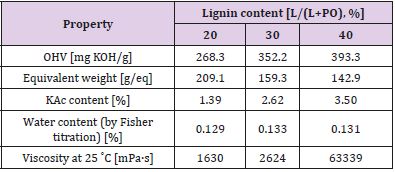
Liquid lignopolyols were obtained by oxypropylation of the lignin fraction in accordance with anionic polymerization in the presence of potassium hydroxide as a catalyst in high pressure laboratory scale (1 L) PARR reactor equipped by an oil thermostat [14]. The calculated amount of propylene oxide (PO), lignin (L) potassium hydroxide (KOH, 5% on DM of lignin) were loaded into the reactor, which was sealed, stirred at room temperature during 40 minutes then heated under stirring using oil thermostat with external round to 160-180˚C when the exothermic process has started. The external heating was turned off and the reaction proceeded in spontaneous autothermic regimes. Due to PO uptake on the conversion pressure in the reactor dropped to a value closed to atmospheric pressure.
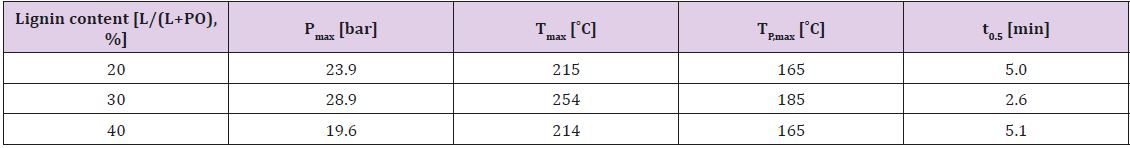
The temperature inside of the reactor was first increased significantly and then lowered due to dissipation of heat in the environment. After cooling, the KOH was neutralized by acetic acid and the product was treated during 2 hours in vacuum rotation evaporator to eliminate the water formed at neutralization of KOH. Dark brown viscous liquids (lignopolyols) completely free of solids were obtained in all cases. The lignin content (L/L + PO) in the reaction was between 20-40%. The compositions of reaction mixtures used for investigation are presented in Table 2. Oxypropylation parameters were adjusted based on the lignin content in the reaction mixture Table 3. Adjustable parameters included maximal pressure in the reactor (Pmax), maximal temperature (Tmax), the temperature at which the exothermic process was started (TP,max) and time necessary for a two-fold decrease of maximal pressure in reactor (t0.5) [15].
Table 3: Dependence of oxypropylation parameters on lignin content in the reaction mixture. Values for the maximal pressure in the reactor (Pmax), maximal temperature (Tmax), the temperature at which the exothermic process was started (TP,max) and time necessary for a two-fold decrease of maximal pressure in the reactor (t0.5) are presented.
Polyurethane Foam Formulation and Characterisation
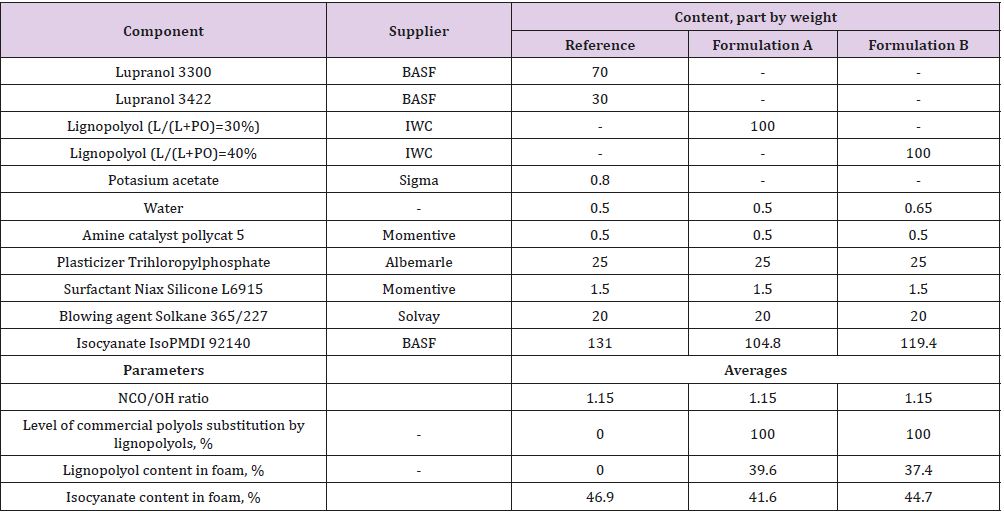
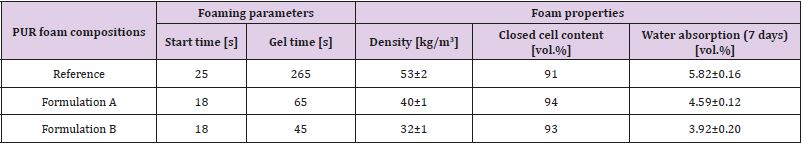
PUR foam compositions were formulated by a complete replacement of commercial glycerol-based polyether and sorbitol based polyether with lignopolyols. All foam demonstrators were obtained by the free rising method [15]. Composition of PUR foam formulations are listed in Table 4. In Formulation B, an increase amount of water (as a chemical blowing agent) was added due its high lignin content (40%) and low density (~30 kg/m3) to ensure the stability PUR foams without shrinkage. The amount of polymeric diphenylmethane diisocyanate (PMDI, NCO 31.5%) in each composition is corrected by using the isocyanate index (NCO/OH ratio) of 1.15. All foams were obtained by the free rising method[13]. The necessary amount of PMDI was added to the mixture of all components excluding isocyanate and mixed for 15 s at the rotation speed of 1750 rpm and then poured into plastic bags of different volume (0.5 l to 5 l) for foaming. Lignin-based polyols PU foams are made by reacting polyols with diisocyanates. The hydroxyl groups of substituted polyols react with the isocyanate groups to form irreversible urethane linkages. Blowing agents (CO2) and additives, such as flame retardants, are necessary to produce the foam’s cellular structure and confer the desired properties. The foams samples were tested after one week of storage. Apparent density, closed cell content, water absorption was determined according to ISO 845:2009, ISO 4590:2003 and ISO 2896:2001, respectively. The compression strength (σ) and Young’s modulus at compression (E) of PUR foams were performed in parallel (x) and perpendicular (z) to foaming directions by testing machine Zwick/Roell Z 100 according to LVS EN ISO 844:2009 standards. However, to correctly compare the strength and modulus of PUR foams the values were normalized(n) to an average density of 40 kg/m3. Here, it was also assumed that in the density range of 32-52 kg/m3 dependence of foams characteristics on their density was directly proportional for each PUR matrix [16]. Normalisation will remove the dependence of the density on compression characteristics of material.
Table 4: PUR foams compositions for different formulations. Reference formulation is using commercial polyols and Formulation A and Formulation B lignin-based polyols.
Results and Discussion
The physicochemical characteristics of synthesised lignopolyols are summarised in Table 5. Results show that the increased amount of METNIN™ lignin oligomer in the reaction mixture during oxypropylation process increases the hydroxyl value (OHV), potassium acetate (KAc) content and viscosity. Hydroxyl values of polyols used in rigid PU foams formulation are in the range 300-800 mg KOH/g and viscosity should be below 300000 mPa·s (at 25˚C) [17,18]. Lignopolyol samples containing 30% and 40% of lignin meet these requirements. Thus, these two lignopolyol samples were selected to be applied in the production of PUR demonstrators. Compared to reference formulation, a significant decrease both in the start time and the gel time of PUR foaming was observed for formulations containing lignopolyols (Table 6). This indicates that lignopolyols were more reactive towards isocyanate compared to the commercial polyethers presented in reference formulation. In addition, the lignopolyol with the higher lignin content showed higher reactivity in the PUR foam system.
Table 6: The foaming parameters, apparent density, closed cell content and water absorption for PUR foams.
The higher reactivity of PUR foam compositions containing
lignopolyols compared to reference formulation resulted to a higher
rate of heat release, a higher temperature in foaming block and as
a sequence the higher volume that occupied the volatiles formed
in the result of water reaction with PMDI (i.e. carbon dioxide) and
physical blowing agents (i.e. Freon) evaporation. As the result,
the apparent density of Formulation A was lower in comparison
with less reactive reference PUR foam composition. In the case of
Formulation B, the lower density was achieved by the increased
water content (0.5 versus 0.65) in the formulation (Table 4). Lower
density is beneficial as it is known that heat insulation properties of
PUR foams are deteriorating with with increased foam density[18].
No traces of foams shrinkage were observed with any of the
formulations, even with Formulation B with very low density.
Results show that over 90% closed cell content was achieved
with all PUR foam formulations (Table 6). The high closed cell
content indicates that crosslinking reactions leading to the
polymeric network strength development and subsequent process
of gaseous phase liberation were in the balance to reach optimal
closed cell structure [18]. This is one of the major requirement
influencing the heat isolation properties of PUR foams.
Water absorption of PUR foams is another parameter influencing
the level of heat isolation characteristics, with a lower water uptake
enabling prolonged exploitation duration to moist conditions [18].
The water absorption values for PUR foams containing a lignopolyol
were lower than those of the reference sample, indicating a more
hydrophobic character of the former (Table 6). In addition, the
water uptake decreases with the increasing lignin content in the
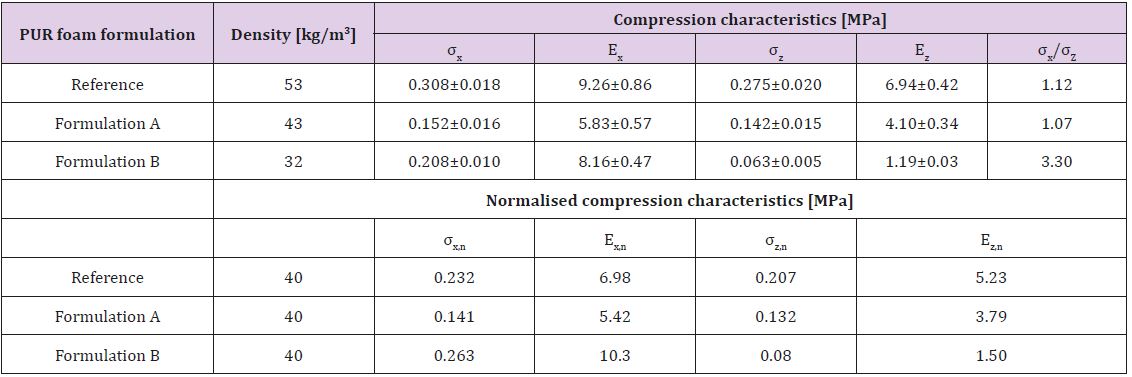
polyol. Compression characteristics of PUR foams are shown in
Table 7. The deformation characteristics of the PUR foam are
dependent on its density as well as on the chemical composition
and structure of polymeric matrix [18].
The apparent density of foams obtained varied in the range
32-49 kg/m3 that meet the requirements for PUR foams usable for
heat isolation in buildings (normally ranges between 30 to 45 kg/
m3) [19]. In all samples, deformation characteristics in foaming
direction exceed those in the direction perpendicular to foaming
(Table 7). This is explained by the elongation of cells foams in
foaming directions, leading to the appearance of anisotropy of
material characteristics. The anisotropy of strength and modulus
was the most pronounced for Formulation B (40% of lignin in
lignopolyol). The compression characteristics in the foaming
direction of PUR foam using Formulation B, which are the dominant
property of the PUR foam, are higher compared to reference
formulation and Formulation B. On the other hand, the opposite is
true for the compression characteristics in perpendicular direction.
Table 7: Compression characteristics of PUR foams., i.e. compression strength (σ) and Young’s modulus at compression (E) in parallel (x) and perpendicular (z) to the foaming direction. Also shown are the corresponding normalized (n) values.
One explanation for anisotropy behaviour could be the lower
NCO conversion at the gel moment in lignopolyol due to its high
functionality. As the result, a significant amount of heat was
evolved after gel formation when the fluidity of the foaming
system decreases dramatically and the growing of foam proceeded
predominantly in a vertical direction. This process leads to
deformation of closed cell form from spherical to ellipsoidal.
Results show that the compression characteristics of PUR foams on
the basis of lignopolyols with 30% of lignin content (Formulation
A) in both directions were lower in the comparison with reference
formulation- , whereas for the higher lignin content (Formulation
B) the compression characteristics exceeded those of the reference
-. In any case, the density and compression characteristics of both
lignopolyol containing foams meet the requirement for PUR foams
suitable for producing heat isolation in a building.
In conclusion, the case study presented here demonstrates
the successful synthesis of lignin based polyols with high lignin
content using METNIN™ lignin oligomer and the subsequent
application of the lignopolyol as a full replacement of commercial
polyol in PUR foam demonstrator. In addition, the selected
technical specifications of the rigid PUR foams are within the
typical industrial requirements. In some cases, lignopolyol shows
improved performance. However, for the full commercial validation,
additional properties such as dimension stability at enhanced
temperature, heat resistance, thermo-oxidative stability and fire
resistance have to be determined. This is in work in progress and
will be presented in further communications.
Acknowledgement
The funding from the Bio Based Industries join Undertaking under the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 792061 (SWEETWOODS) is acknowledged. It is recognized that results are based on the contractual work between MetGen Oy (Kaarina, Finland) and Latvian State Institute of Wood Chemistry (Riga, Latvia) by Prof. Galina Telysheva’s group.
Conflict of Interest
The authors declare no conflict of interest.
References
- S Xie, Arthur J, Ragauskas, JS Yuan (2016) Lignin Conversion: Opportunities and Challenges for the Integrated Biorefinery. Ind Biotechnol 12(3): 161-167.
- DS Bajwa, G Pourhashem, AH Ullah, SG Bajwa (2019) A concise review of current lignin production, applications, products and their environmental impact. Ind Crops and Products 139.
- NV Gama, A Ferreira, A Barros Timmons (2018) Polyurethane Foams: Past, Present, and Future. Materials 11(10).
- Alinejad M, Henry C, Nikafshar S, Gondaliya A, Bagheri S, et al. (2019) Lignin-Based Polyurethanes: Opportunities for Bio-Based Foams, Elastomers, Coatings and Adhesives. Polymers 11(7): 1202-1223.
- Z Sun, B Fridrich, A de Santi, S Elangovan, K Barta (2018) Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chemical Reviews 118(2): 614-678.
- Ł Klapiszewski, TJ Szalaty, T Jesionowski (2018) Depolymerization and Activation of Lignin: Current State of Knowledge and Perspectives. Intech Open.
- J Becker, C Wittmann (2019) A field of dreams: Lignin valorization into chemicals, materials, fuels, and health-care products. Biotechnol Adv 37(6): 107360-107384.
- Veera Hämäläinen, Toni Grönroos, Anu Suonpää, Matti Wilhem Heikkilä, Bastiaan Romein, et al.(2018) Enzymatic process to unlock the lignin value. Front Bioeng Biotechnol 6(20): 1-10.
- metgen.com
- sweetwoods.eu
- J Sameni, S Krigstin, D dos Santos Rosa, A Leao, M Sain (2014) Thermal Characteristics of Lignin Residue from Industrial Processes. BioResources 9(1): 725-737.
- M Lauberts, L Lauberte, A Arshanitsa, T Dizhbite, G Dobele, et al. (2018). Structural transformations of wood and cereal biomass component induced by microwave assisted torrefaction with emphasis of extractable chemicals obtaining. J Anal Appl Pyrolysis 134: 1-11.
- GF Zakis (1994) Functional Analysis of Lignins and their Derivatives. TAPPI Press.
- B Berrima, G Mortha, S Boufi, E El Aloui, MN Belgacem (2016) Oxypropylation of Soda Lignin: Characterization and Application in Polyurethane Foams Production. Cellulose Chem Technol 50 (9-10): 941-950.
- G Sung, H Choe, Y Choi, Jung Hyeun Kim (2018) Morphological, acoustical, and physical properties of free-rising polyurethane foams depending on the flow directions. Korean J Chem Eng 35(4): 1045-1052.
- X Cao, LJ Lee, T Widya, C Masockso (2005) Polyurethane/clay nanocomposites foams: processing, structure and properties. Polymer 46(3): 775-783.
- P Furtwengler, L Avérous (2018) Renewable polyols for advanced polyurethane foams from diverse biomass resources. Polymer Chemistry (32): 4258-4287.
- JM Buist (1978) Development in polyurethane. 1 Applied Science Publishers 1-290.
- react-ite.eu

 Research Article
Research Article