Abstract
Primary open-angle glaucoma (POAG) is an important type of glaucoma, and the pathogenesis is not fully understood. The high intraocular pressure (IOP) is considered as one of the most risk factors. Clinical evidence indicates that increased intraocular pressure is a function of elevated resistance to drainage of the trabecular meshwork (TM) outflow pathways. However, there is still no consensus about exact location of the increased outflow resistance of aqueous humor, and the mechanism is not perfect. The microstructure information of the TM outflow pathways is of great significance for analyzing the outflow resistance distribution of the outflow pathways. Therefore, it is particularly important to strengthen the morphological study of the outflow resistance of aqueous humor in the TM outflow pathways. In this study, the two-photon confocal imaging technique was used to image the TM outflow pathways, and image data of the longitudinal section of the TM outflow pathways were obtained. The image segmentation method based on deep learning was used to further study and analyze the trabecular meshwork, which laid morphological basis for the clinical diagnosis study.
Keywords: Glaucoma; Trabecular Meshwork; Porosity; Deep Learning
Abbreviations: POAG: Primary Open-Angle Glaucoma; IOP: Intraocular Pressure; TM: Trabecular Meshwork; AH: Aqueous Humor
Introduction
Primary Open-Angle Glaucoma (POAG) is the most common type of glaucoma worldwide, accounting for about 70% of the total number of glaucoma cases [1]. Primary open-angle glaucoma is an important type of glaucoma, its pathogenesis is not clear, and one of the main factors for its occurrence and development is the increase of pathological intraocular pressure. In addition, experimental evidence suggests that the central place of the resistance of aqueous drainage is the vicinity of trabecular meshwork (TM), including the endothelium of SC [2]. It is postulated that the increase of outflow resistance is provided by the functional changes or structural abnormalities in TM, which leads to ocular hypertension associated with numerous cases of POAG [3]. At present, it is believed that the reason for the increased IOP in primary open-angle glaucoma is the increased outflow resistance of aqueous humor (AH), especially the increased outflow resistance of trabecular meshwork. The trabecular meshwork is embedded in the scleral sulcus and is a porous mesh structure composed of interconnected trabecular beams. In view of the small size and complex structure of the trabecular meshwork, imaging technology needs to achieve a micron-level spatial resolution to accurately obtain the structural information of the trabecular meshwork; on the other hand, the trabecular meshwork is located below the corneoscleral limbus and has a certain depth. The surface sclera scatters greatly during imaging, and the imaging device needs to have a certain detection depth. As mentioned above, the two-photon microscopic imaging technology has sub-cell-level resolution and its ability to obtain tissue structure and function information, which has attracted more and more attention in experimental-based biomedical imaging [4,5]. At present, it is believed that the two-photon imaging technique is a relatively effective research tool for obtaining the trabecular meshwork and Schlemm’s canal morphology. For example, Gonzalez et al.
Used the two-photon excitation fluorescence method to image the trabecular meshwork tissue of the human eye and obtained relatively clear trabecular meshwork tissue autofluorescence imaging data. The pore structure is due to the lack of collagen fibers [4,6]. Masihzadeh et al. Placed the anterior chamber angle lens in front of the confocal imaging device to image the pig’s eye trabecular meshwork tissue from different angles and stitched the obtained image data into a 2.5D stereo morphology map [7]. Zhang et al. [8] successfully acquired image data such as Schlemm’s canal, collecting canal, and aqueous venous veins based on spectral two-photon microscopy, and obtained a remote model of the local trabecular meshwork channel through 3D reconstruction. In the modern medical research work, the medical image plays an increasingly important role. In the process of studying the structure of trabecular meshwork, it is very important to accurately sketch the structure of trabecular meshwork and the surrounding normal tissues. The traditional method is for doctors to manually mark the computed tomography images, which is time-consuming and labor-intensive and highly dependent on the doctor’s experience [9]. Therefore, we hope to use image segmentation technology based on deep learning to help doctors sketch trabecular meshwork tissue images. Using computer aided technology to improve the accuracy and efficiency of the work. Therefore, this paper researches the trabecular meshwork image segmentation method based on deep learning. Finally, based on U-Net network structure, the trabecular meshwork segmentation model is successfully constructed to complete the task of trabecular meshwork organization segmentation, which laid a morphological foundation for the subsequent research on aqueous humour dynamics and laid a morphological basis for the clinical diagnosis study. The accuracy rate of pixel in model test is 99%, and good training effect is obtained.
Materials and Methods
Tissue Preparation
Studies were performed on ocular tissues harvested from adult SD rats (weighing 320–350g) of either sex provided by the animal department of Capital medical University (IACUC: AEEI-2013-x-123). The eyes were immediately enucleated after euthanasia and immersed in phosphate buffered saline (PBS, pH 7.4) containing 8 g L-1 sodium chloride, 2.16 g L-1 sodium phosphate dibasic heptahydrate and 0.2 g L-1 potassium phosphate monobasic. A total of 16 eyeballs were divided into three groups. Half of the rats were prepared for imaging under the condition of ocular hypertension (group B), and the other group was used as the control group (group A) for imaging in situ. The system was a functional method of that described previously [10].
Two-Photon Microscopy Imaging
Image capturing was performed using a TPM system at the Research Lab for Biomedical Optics and Molecular Imaging (SIAT, CAS, CHN) [11]. The excitation laser source (900nm) was provided using a tunable mode-locked Ti: Sapphire laser (Coherent Inc., Santa Clara, CA, USA) emitting a train of approximately 140-fs width pulses at a repetition rate of 80-MHz. The excitation beam (Ti: Sapphire laser) was focused on the sample, and the backscattered signal was collected using a 20×/1.00 NA water-immersion objective (Olympus Inc.). Finally, the z-stack images were gathered and processed using a custom-designed Labview program.
Image Analysis
This research builds the segmentation model of trabecular meshwork based on U-Net network, and realizes the segmentation of trabecular meshwork image through pixel-by-pixel classification of the image. First, use the labeling tool to label the trabecular meshwork image and generate the json format of the corresponding image. The result of execution will generate a folder corresponding to the picture, which includes four files: img, info, label, label_viz. The next step is to process the generated image file. After executing the files rename.py, color.py, and resize2.py, an annotated image of the corresponding image will be generated, and the annotated image will be converted to a grayscale image. For calculation of porosity, we get segmentation threshold from gray histogram and calculated the ratio of pores’ area in valid image.
Results
SHG Imaging of Ocular Tissues
The microstructure information of the trabecular meshwork channel is the morphological basis for studying the flow of aqueous humor. In view of the small spatial size and complex structure of the trabecular meshwork, imaging technology requires a spatial resolution of the order of micrometers to accurately obtain the structural information of the trabecular meshwork; on the other hand, the trabecular meshwork is located below the limbus of the cornea and has a certain depth. During tissue imaging, the sclera scatters a lot, and the imaging device needs to reach a sufficient imaging depth to achieve cross-scleral imaging. The data in this study is based on a self-built two-photon confocal system. The laser of the two-photon confocal imaging system is irradiated vertically to the limbus area of the outer surface of the rat’s eyeball, and the trabecular meshwork channel tomogram is collected through the opaque scleral tissue. The limbus of the cornea is defined as the transition zone between the cornea and the sclera. It is translucent and has no clear boundary on the lateral wall of the anterior chamber angle. In order to locate the specific position of the cornea and sclera, we first performed two-photon microscopy imaging study on the cornea and sclera tissue of healthy rats without fixation and obtained the second harmonic imaging data of normal rat cornea and sclera tissue. The results show that compared with the sclera, the corneal fiber arrangement seems to be more regular, and the second harmonic signal exhibits a repetitive interlocking structure. By distinguishing the second harmonic signal of the cornea and scleral tissue, the sclera and the cornea can be distinguished from the interactive connection area and help to identify the trabecular meshwork and surrounding tissues under the cornea limbus.
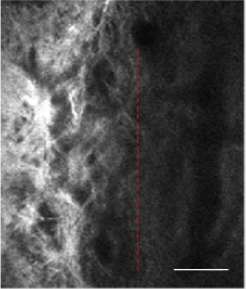
(Figure 1) illustrate the region of the TM in the normal condition. According to the imaging principle of TPM, the zones absent of signals agree with the aqueous-filled spaces within the AOS, and the collagen fibres of the TM can be clearly seen. The TM beams seem to be heterogeneous with depth increases. Additionally, it is easy to identify the superficial meshwork, which is a filter comprising a network of connective tissue. There are dark regions, which are shown as gaps between these beams and which allow the drainage of AH.
Figure 1: The image was taken at a depth of 200μm below the corneoscleral limbus. The left of red dotted area is the trabecular meshwork channel tissue.
Implementation of Image Segmentation Code of Trabecular Meshwork Based On U-Net Network
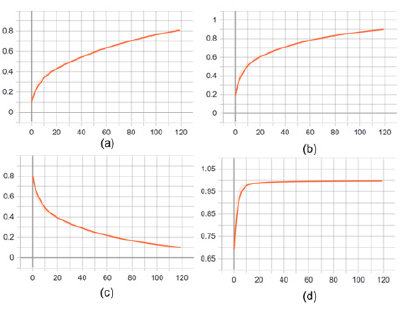
This paper builds a segmentation model of trabecular meshwork on the basis of U-Net network, and realizes the segmentation of trabecular meshwork image through pixel-by-pixel classification of the image. Due to the simpler and more intuitive advantages of keras to build a model, the unet model implemented here can not only deal with dichotomous problems, but also multi-class segmentation problems. The partial results of image segmentation of trabecular meshwork are shown in Figure 2. At present, there are many risks in the treatment of glaucoma, so it is urgent to find new methods to restore or replace the damaged trabecular meshwork. Therefore, it is very important to study the structure of trabecular meshwork. The image segmentation method based on deep learning was used to further study and analyze the trabecular meshwork, which laid a morphological foundation for the subsequent research on clinical diagnosis. The default parameter optimization model of Adam algorithm is used in the research. The initial learning rate of the model is 0.001, and the learning rate is dynamically adjusted during the training process. The batch size batch is set to 6. During the training process, the validation set loss passes through 119 steps, 42 epochs no longer decline and stop. The IoU(Intersection over Union), Dice coefficients, cross-entropy loss curve and acc change curve obtained from the Tensorboard visualization training process are shown in Figure 3, As shown in Figure 3, the light-colored lines in the figure are the original data curves, and the dark lines are the smoothed curves. The performance indicators used for image segmentation are IoU and Dice Coefficient. IoU is an index used to evaluate segmentation performance. The calculation method is: IoU = overlapping part/(real label + actual segmentation result).
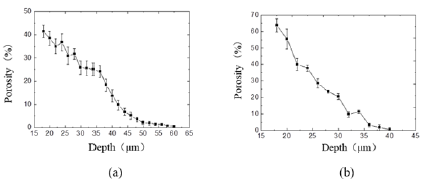
The Dice Coefficient is used to evaluate the similarity between the segmentation result and the real label. Dice Coefficient = 2*predicted correct result/(predicted result + actual result). From Figure 2d, after 119 steps (42 epochs), the IoU is 0.8086, the accuracy acc is 0.9977, and the loss and dice coefficients are 0.101 and 0.8976, respectively, indicating that the model segmentation accuracy is more accurate and the training effect is better 3.3 Porosity of trabecular meshwork. When the TM inner layer was set at 0 μm, the porosity of the TM decreased with its increasing depth (in the flow direction). As shown in Figure 4a, the porosity of the TM with normal IOP was slowly decreased. While under the condition of elevated IOP Figure 4b, it was descending quickly. Additionally, the porosity of the TM in rat eyes with high IOP was less than that in the normal IOP group from the depth of 30 μm to the depth of 60 μm. Under the condition of elevated IOP (7.98 kPa), the porosity of the TM in the superficial region of the TM was rapidly decreasing from 0.65 to 0.31 and decreased from 0.31 (depth 30 μm) to 0.05 (depth 40 μm) in the deeper TM tissue adjacent to the SC. The TM tissue in the high IOP group exhibited the structures of compressed collagen fibres, and the total thickness of the TM decreased compared with the controls. It is indicated that the TM tissue can be collapsed by fusing into surrounding tissues due to the function of elevated IOP. In addition, the findings demonstrated that the changes in the TM tissue in pressurized eyes are large enough to lead to the increased outflow resistance.
Figure 3: Performance index and evaluation index of image segmentation.
a. IoU coefficient variation curve.
b. Dice coefficient variation curve.
c. Cross entropy loss change curve.
d. Accuracy rate change curve.
Figure 4: Curve of trabecular meshwork porosity with image depth at different intraocular pressures.
a. Under the condition of normal IOP
b. Under the condition of elevated IOP (7.98 kPa).
The meshwork inner layer corresponding to superficial TM was set at 0μm for the calculation of TM porosity.
Discussion
In recent years, researchers have conducted imaging studies on trabecular meshwork channel tissues based on two-photon imaging technology, which has been limited to obtaining only partial trabecular meshwork channel image data, namely, the proximal end of the trabecular meshwork channel or trabecular meshwork. Research on the trabecular meshwork and its surrounding tissue morphology has always been the focus of scholars’ attention, but the understanding of the microstructure of the trabecular meshwork channel is still relatively limited. In this study, two-photon confocal imaging technology was used to image the trabecular meshwork and its surrounding tissues were obtained. In this study, the infrared wavelength of 800 nm inevitably leads to broad melanin autofluorescence, and previous studies of TPM imaging in TM regions have been discussed [12,13]. Our work extends the study of TM imaging in enucleated rat eyes using the improved techniques [8,11]. A longer excitation wavelength of 900 nm was effective in reducing scattering of the scleral tissue and minimizing autofluorescence from melanin granules, and the resolution increased (up to 0.5 μm). Thus, we obtained detailed structures of AOS, including TM, adjoining SC and CC. The corneoscleral limbus is defined as the transition zone between the cornea and the sclera. It is translucent and has no clear boundary on the lateral wall of the anterior chamber angle. Because the boundaries of the trabecular meshwork area are difficult to distinguish, the image segmentation of trabecular meshwork through deep learning is of great significance. We determined the porosity of TM using the TPM sequence images. It has many correlations with TM porosity, image depth and IOP. In addition, we demonstrate that when the depth of the TM in the flow direction is deeper, the TM porosity will be greater, which is in close proximity to the morphology of the TM. Most importantly, assessing the structures of the TM in a pressurized environment accurately is exceedingly significant and can provide sufficient information for the pathogenesis of glaucoma. With the rapid development of medical imaging technology, the analysis of microscopic images and digital pathological images is an important way of medical research [14]. Observing and analyzing a large number of medical images by scientific researchers is timeconsuming and labor-intensive. Therefore, the use of computer technology to assist research can help improve efficiency, reduce costs, and accelerate research progress. Medical image segmentation is a key step in the automated analysis of medical images. This article is based on deep learning technology to study the image segmentation of trabecular meshwork, and builds a deep convolutional neural network for image segmentation on the basis of U-Net algorithm [15]. The automatic segmentation of medical images organized by trabecular meshwork is hoped to provide some inspiration for further realization of medical image recognition.
Conclusion
This chapter introduces the U-Net network structure and the specific software and hardware environment of this experiment. The trabecular meshwork segmentation model is constructed on the basis of U-Net network, and the trabecular meshwork image segmentation is successfully realized. In this experiment, the default parameter optimization model of Adam algorithm is adopted, and the overall accuracy is high. The experimental results are only valid for this experimental data. On the other hand, compared with traditional methods, image segmentation algorithms based on deep learning have the advantages of high efficiency and low cost, but in some cases traditional image segmentation methods still have unique advantages. In order to further improve the effect of image segmentation, you can try to combine traditional segmentation algorithms with deep learning-based algorithms for research. The morphological structure of the trabecular meshwork, the adjacent tube structure and the Schlemm’s canal with different depths were analyzed from different directions, which laid a morphological basis for the clinical diagnosis study.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 31570952, 10802053, 81471702), the Natural Science Fund for Colleges and Universities in Jiangsu Province (19KJB310024), Startup Fund for Youth Talent in Xuzhou Medical University (D2019009), Science and Technology Development Program of Xuzhou (KC19194).
References
- Tham YC, Li X, Wong TY, Quigley HA, Aung T, et al. (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121(11): 2081-2090.
- J Buffault, A Labbé, P Hamard, F Brignole, C Baudouin, et al. (2020) The trabecular meshwork: Structure, function and clinical implications. A review of the literature. Journal Franais d'Ophtalmologie 43(7): 1-14.
- Johnson M (2006) What controls aqueous humour outflow resistance? Experimental eye research 82(4): 545-557.
- Gonzalez J M, Ammar M J, Ko M K, Tan J C (2016) Optimizing two-photon multiple fluorophore imaging of the human trabecular meshwork. Mol Vis 22: 203-212.
- Doi A, Oketani R, Nawa Y, Fujita K (2018) High-resolution imaging in two-photon excitation microscopy using in situ estimations of the point spread function. Biomedical Optics Express 9(1): 202-213.
- Gonzalez JM, Heur M, Tan JCH (2012) Two-photon immunofluorescence characterization of the trabecular meshwork in situ. Invest Ophthalmol Vis Sci 53(7): 3395-3404.
- Masihzadeh O, Ammar DA, Kahook MY, Emily G, Tim CL (2013) Direct trabecular meshwork imaging in porcine eyes through multiphoton gonioscopy. Journal of Biomedical Optics 18(3): 36009.
- Zhang Xianzeng, Liu Nenrong, Peng UM, Sio HP, Mang I, et al. (2016) Three-dimensional segmentation and quantitative measurement of the aqueous outflow system of intact mouse eyes based on spectral two-photon microscopy techniques. Invest Ophthalmol Vis Sci 57(7): 3159-3167.
- Xu X, Lu Q, Yang L, Hu S, Chen D, Shi Y (2018) Quantization of fully convolutional networks for accurate biomedical image segmentation. IEEE/CVF Conference on Computer Vision and Pattern Recognition 2018: 8300-8308.
- Zhang J, Ren L, Mei X, Xu Q, Zheng W, et al. (2016) Microstructure visualization of conventional outflow pathway and finite element modeling analysis of trabecular meshwork. Biomedical Engineering Online 15: 162.
- Zheng W, Wu Y, Winter P, Robert F, DD Nogare, et al. (2017) Adaptive optics improves multiphoton super-resolution imaging. Nature methods 14: 869-872.
- Masihzadeh O, Ammar DA, Kahook MY, Gibson EA, Lei TC, et al. (2013) Direct trabecular meshwork imaging in porcine eyes through multiphoton gonioscopy. Journal of biomedical optics 18(3): 036009.
- Masihzadeh O, Lei TC, Ammar DA, Kahook MY, Gibson EA (2012) A multiphoton microscope platform for imaging the mouse eye. Molecular vision 18(187-190): 1840-1848.
- Badrinarayanan V, Kendall A, Cipolla R (2017) Segnet: A deep convolutional encoder-decoder architecture for image segmentation. IEEE Transactions on Patter Analysis and Machine Intelligence 39(12): 2481-2495.
- Xiao Z, Liu B, Geng L, Zhang F, Liu Y (2020) Segmentation of Lung Nodules Using Improved 3D-UNet Neural Network. Symmetry 12(11): 1787.

 Research Article
Research Article