ABSTRACT
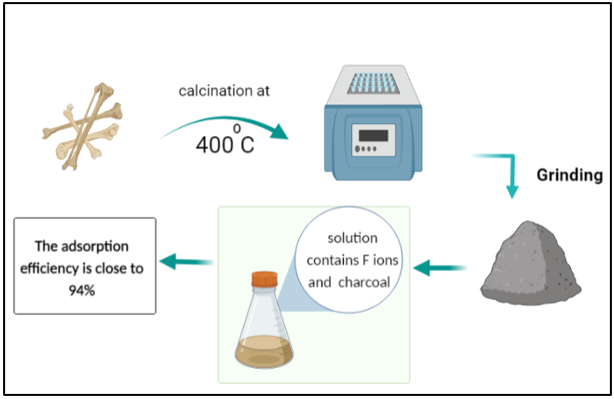
The research on clean water production is of critical importance worldwide. Excess fluoride pollutant has direct correlation with bones deformation. Bio-filters are of multilateral advantages. This study is focused on the physical treatment to prepare lowcost biochar adsorbents made from cow bone. The produced bio-filter was tested for fluoride removal from aqueous solutions containing control amounts of fluoride. The bone sample was calcined at 400 °C. Scanning electron microscopy, energy dispersive X-ray analysis, and ionic chromatography were performed to characterize the filtration performance of the charcoal adsorbent. The adsorption effectiveness of fluoride was estimated at 94%. The proposed charcoal bio-filter is economically and environmental advantageous, given its lack of energy consumption. It also provides health benefits. Furthermore, the overall performance of this bio-filter could be enhanced in the future by using nanocomposites.
Keywords: Fluoride; Cow Bone Biochar; Economy; Health; Environment; Drinking Water; SEM; EDX; IC; Nanocomposites
Abbreviations: SEM: Scanning Electron Microscope; EDX: Energy Dispersive X-ray; PPM: Parts-Per-Million
Introduction
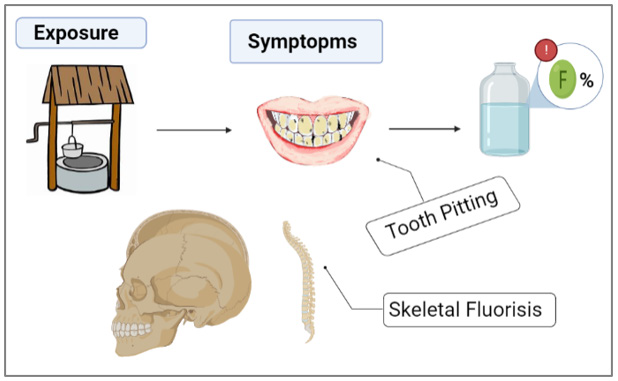
One of the main importance of water filtration is to prevent water-related illnesses and diseases. To this day, various explored methods were used in the remediation of water of different types of contaminants such as flocculation [1,2], coagulation [3], solventextraction [4], co-precipitation [5], precipitation [6], ion-exchange [7], photo catalysis [8], adsorption-desorption [9], reverse-osmosis [10], nano-membrane filtration [11]. The adsorption technique is considered one of the attractive and commercial options to eliminate of the most pollutant substances whether macro- or micro- organic/inorganic ions from water, due to its simplicity of steps and high activity. Moreover, the purification of water by using adsorption technique has been applied to several matter as absorbent such as agricultural residues and industrial residues, and biomaterials wastes, which are modified and applied in biosorption of contaminants from water [7,9-12]. Fluoride is an ionic form of fluorine and can be found in food and numerous sources of drinking water. It can be also purchased as a dietary supplement [12]. Many kinds of toothpastes contain fluoride because these ions serve as an armor against tooth decay [13]. Approximately 80% of the fluoride taken orally is absorbed. Humans retain around 50% of the fluoride they consume, and most of this amount is deposited in teeth and bones (Graphical Abstracts 1 & 2). The remaining 50% is excreted in the urine.
However, young children can retain an especially high percentage of the consumed fluoride, because their bones and teeth absorb more fluoride than those of adults [14]. The principle and mechanism of pollutants removal by adsorption technology from different types of water based on forms layer of condensate pollutants (called adsorbate) which is migrated from aqueous solution to the surface of solid (called adsorbent) as either in the form of liquid-solid interface [11,15]. Excess fluoride is harmful to human health. Groundwater wells worldwide have been reported to contain water with fluoride concentrations exceeding the acceptable level of 1.5 mg/L. This fact was attributed to local ground stones exhibiting high percentages of fluoride in their composition [14]. Excessive fluoride consumption can lead to several health issues, such as skeletal fluorosis, a disorder marked by bone and joint pain as well as joint tenderness. The overconsumption of fluoride during the formative years of tooth enamel can also lead to dental fluorosis, which leads to tooth discoloration, and/or tooth pitting [15]. Many researchers have attempted to develop water filtration strategies to lower the levels of fluoride or metals that contaminate water [16]. Biomaterials have shown great promise in water filtration because of their environmentally favorable properties, high filtering efficiency, and low cost [17].
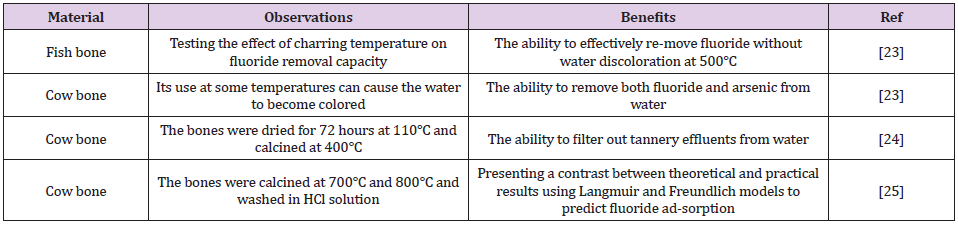
Charcoal, which may be manufactured from a variety of biomaterials, is one of the most commonly used bio-filters. Water contaminated with heavy metals can be treated using different processes, which include ion exchange, precipitation, reverse osmosis, catalysis, coagulation, and adsorption [18,19]. Heavy metal adsorption is dependent on the nature of the adsorbents, which can be composed of natural or man-made mate-rials including clay [10], sludge [11], industrial waste, activated carbon, and plants [12]. Bone char is an adsorbent made up of 90% calcium phosphate and 10% carbon. It can be created using one of the two methods: treatment with chemicals and physical treatment leading to carbonization of bones [20,21]. Cow bone char is com-monly used to purify water contaminated with heavy metals, as shown in (Table 1). The aim of the current study was to produce a lowcost, effective, and environmentally friendly biochar adsorbent from cow bone through physical activation (carbonization), and subsequently use it to remove fluoride from the polluted water [22]. Furthermore, it was also proposed to enhance this bio-filter by adding nanocomposites. Nanocomposites have very high surfaceto- volume ratios, which makes them ideal components for the adsorption process [23,24].
Experiments
Cow bones were collected and processed by rinsing them several times with hot water to remove residual muscles and other joint tissues. Then the bones were allowed to dry in the open air. The dried bones were calcined in a furnace for 1 h at 400°C. The resultant charcoal adsorbent was crushed using a gate mortar and stabilized for further testing. It can be stated that the filters were produced with little to no expenses. A stock solution of CaF2 with a 100-ppm concentration of fluoride was prepared to test its adsorption in the cow bone sample. First, 1 g of charcoal was added to the 100 mL solution in a separate beaker, which was then placed in a shaker operated at room temperature and a 251 min-1 frequency for 5 h. Next, on the same day, the solution containing cow bone charcoal was filtered from impurities and charcoal leftovers using filter paper to prepare a sample for ionic chromatography (IC) [25]. IC was utilized for determining the concentrations of calcium and fluoride ions in the prepared CaF2 solution. Ion chromatographs can measure concentrations of important anions such as fluoride, chloride, nitrate, nitrite, and sulfate, as well as major cations such as lithium, sodium, ammonium, potassium, calcium, and magnesium in the parts-per-million (ppm) range. Ion chromatography can also be used to determine the concentrations of organic acid [26].
The surface structure of cow bone charcoal was studied by using a scanning electron microscope (SEM) (Model: JSM-7100F) at 10000 × magnification [27]. In conjunction with SEM, the energy dispersive X-ray (EDX) analysis technique was used to perform the elemental analysis and chemical characterization of the material. EDX utilizes an electron beam that strikes the surface of a conducting sample (placed under SEM) to determine its elemental content [28].
Results and Discussion
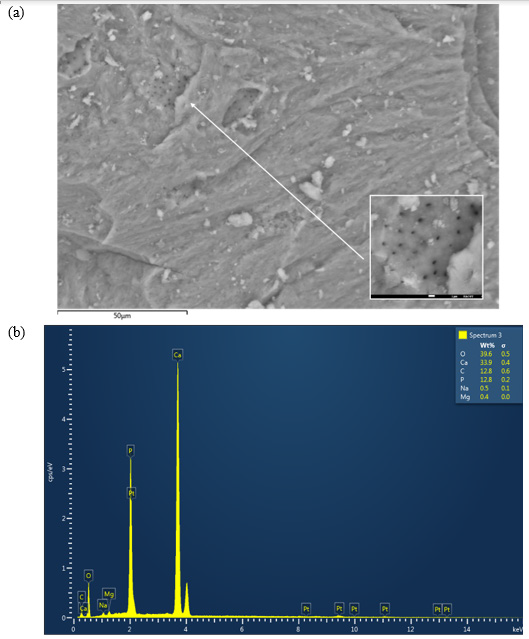
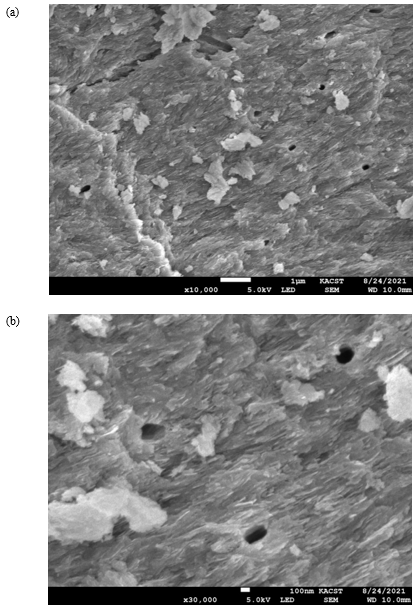
The SEM micrographs of the cow bone biochar explained the sample morphology by analyzing the microstructure of the bone powder, as shown in (Figure 1a). The corresponding EDX graph is presented in (Figure 1b). A close look at (Figure 1a) reveals cracks and irregular surfaces, as expected after grinding, where Vickers hardness can be determined (it is planned to be measured in the upcoming study). Furthermore, SEM micrographs showed that the studied samples had several heterogeneous porous layers, which represent a key feature relevant for fluoride adsorption. The pores appeared to be localized symmetrically in certain regions in the grooves on the surface of the bone. The sample composition revealed by EDX results was as follows: O2, 39.6 wt%; Ca, 33.9 wt%; C, 12.8 wt%; P, 12.8 wt%; Na and Mg, 0.9 wt%.
Figure 1: (a) Scanning electron microscope (SEM) micrograph of the cow bone charcoal shows several groups of symmetrically distributed pores. (b) The corresponding energy dispersive X-ray (EDX) graph of the bone constituents.
The surface morphology of the cow bone is particularly distinctive in terms of the hard appearance and the distribution of pores, which are of critical importance in filtering large species from water (Figure 2). Hence, the surface geometry and the source of adsorbent provided different types of active sites onto surface of adsorbent [29-35] (Table 2).
Figure 2: (a) SEM micrograph of cow bone charcoal shows symmetrically distributed pores on the surface (red arrows) with diameters in the range of several hundred nanometers, as shown in (b).
Conclusion
The examined cow bone charcoal filter achieved maximal fluoride adsorption of 93.6%, which can be attributed to its porous nature. The prepared bio-filter has a very high adsorption capacity and is energy-efficient as it works at room temperature and does not require energy consumption. It means that using cow bone charcoal is a cost-effective filtration technique that should be further investigated to optimize the performance of nanocomposites and to set up measures for its widespread manufacturing.
Supplementary Materials
N/A.
Author’s Contribution
Alasmari, E, M, carried out experiments and engaged in various article-related tasks. Alsharif, S. H. performed the experimental measure-ments. Khayyat, M. M. designed the experiments and prepared the final version of the manuscript.
Funding
This work was performed as part of the joint program of KACST and Mohiba ITHRAA 2021.
Data Availability Statement
The obtained results have been presented in the cur-rent article, and any further details can be provided upon request made to the cor-responding author.
Acknowledgment
The authors would like to thank Dr. Saeed M. Alshehri, the director of MSRI, KACST, for his kind support throughout the project.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Chaudhry F, M Malik (2017) Factors affecting water pollution: A review. J Ecosyst Ecography 7(225): 1-3.
- Lapointe M (2020) Understanding and Improving Microplastic Removal during Water Treatment: Impact of Coagulation and Flocculation. Environmental Science & Technology 54(14): 8719-8727.
- Sillanpää M (2018) Removal of natural organic matter in drinking water treatment by coagulation: A comprehensive review. Chemosphere 190: 54-71.
- Ren X, Xinhe Zhao, François Turcotte, Jean-Sébastien Deschênes, Réjean Tremblay, et al. (2017) Current lipid extraction methods are significantly enhanced adding a water treatment step in Chlorella protothecoides. Microbial cell factories 16(1): 1-13.
- Shabani N (2021) Potential Application of Iron Oxide Nanoparticles Synthesized by Co-Precipitation Technology as a Coagulant for Water Treatment in Settling Tanks. Mining, Metallurgy & Exploration 38(1): 269-276.
- Kalaitzidou K, A Zouboulis, M Mitrakas (2020) Cost evaluation for Se (IV) removal, by applying common drinking water treatment processes: Coagulation/precipitation or adsorption. Journal of Envi-ronmental Chemical Engineering 8(5): 104209.
- Levchuk I, JJR Màrquez, M Sillanpää (2018) Removal of natural organic matter (NOM) from water by ion exchange–a review. Chemosphere 192: 90-104.
- Ahmed SN, W Haider (2018) Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 29(34): 342001.
- Al-Shehri BM (2019) A review: The utilization of mesoporous materials in wastewater treatment. Materials Research Express 6(12): 122002.
- Malaeb L, GM Ayoub (2011) Reverse osmosis technology for water treatment: State of the art review. Desalination 267(1): 1-8.
- Rashidi HR, N Meriam Nik Sulaiman, N Awanis Hashim, C Rosmani Che Hassan, M Redzuan Ramli, et al. (2015) Synthetic reactive dye wastewater treatment by using nano-membrane filtration. Desalination and Water Treatment 55(1): 86-95.
- Brindha KL, Elango L (2011) Fluoride in groundwater: Causes, implications and mitigation measures. Fluoride Prop Appl Environ Manag, pp. 113-136.
- Armour B, Swanson M, Waldman H, Perlman S (2008) A profile of state-level differences in the oral health of people with and without disabilities, in the U.S., in 2004. Public Health Rep 123(1): 67-75.
- O’mullane D, RJ Baez, S Jones, MA Lennon, PE Petersen, et al. (2016) Fluoride and Oral Health Sections. Community Dent. Health 33(2015): 69-99.
- Kim H (1999) Electrical, optical, and structural properties of indium-tin-oxide thin films for organic light-emitting devices. J Appl Phys 86(11).
- Aoba T, Fejerskov O (2002) Defluoridation of water. 13(2): 155-170.
- Yadav K, Sandeep Kumar, Quoc Bao Pham, Neha Gupta, Shahabaldin Rezania, et al. (2019) Fluoride contamination, health problems and remediation methods in Asian ground water: A comprehensive review. Ecotoxicol Environ Saf 182: 109362.
- Minamisawa M (2004) Adsorption behavior of heavy metals on biomaterials. Journal of agricultural and food chemistry 52(18): 5606-5611.
- Gupta SS, KG Bhattacharyya (2011) Kinetics of adsorption of metal ions on inorganic materials: A review. Advances in colloid and interface science 162(1-2): 39-58.
- Brima E, Haris P (2015) Arsenic Removal from Drinking Water using Different Biomaterials and Eval-uation of a Phytotechnology Based Filter. Int Res J Environ Sci 3: 39-44.
- Gunatilake S (2015) Methods of Removing Heavy Metals from Industrial Wastewater. 1(1): 12-18.
- Arora R (2019) Adsorption of heavy metals-a review. Mater Today Proc 18(1): 4745-4750.
- Guerra D, Silva R, Mello I (2013) Adsorption of mercury from aqueous solution by nontronite, As-pergillus niger, and hybrid material. Water Qual Res J Canada 48(2): 155-170.
- Villabona Ortíz A, Tejada Tovar C, R Ortega Toro (2020) Modelling of the adsorption kinetics of chromium (VI) using waste biomaterials. Rev Mex Ing Quim 19(1): 401-408.
- Kanawade S, Gaikwad R (2011) Lead Ion removal from Industrial effluent by using Biomaterials as an Adsorbent. Int J Chem Eng Appl no January, pp. 196-198.
- Mizan A (2018) Preparation of Activated Cow Bone Charcoal for Abatement of Water Pollution caused by Tannery Effluents. RESEARCH ARTICLE PREPARATION OF ACTIVATED COW BONE CHARCOAL FOR ABATEMENT OF.
- Nigri E, Maria Alice P Cechinel, Diego A Mayer, Luciana P Mazur, José M Loureiro, et al. (2017) Cow bones char as a green sorbent for fluorides removal from aqueous solutions: batch and fixed-bed studies. Environ Sci Pollut Res 24(3): 2364-2380.
- Jerome N (2018) Quantitative Characterization of Activated Carbon from Cow, Donkey, Chicken and Horse Bones from Ezzangbo in Ebonyi State, Nigeria. Am J Appl Chem 6(5): 169-174.
- Driscoll J (2005) Gas Chromatography in Environmental Analysis. Environ Instrum Anal Handb, pp. 157-186.
- Jackson P (2000) Ion Chromatography in Environmental Analysis. Encycl Anal Chem, pp. 2779-2801.
- Mohammed A, Abdullah A (2018) Scanning Electron Microscopy (SEM): A Review. Int Conf Hy-draul Pneum 7: 1-9.
- Almousa N, Maha R Alotaibi, Mohammad Alsohybani, Dominik Radziszewski, Saeed M AlNoman, et al. (2021) Paraffin Wax [As a Phase Changing Material (PCM)] Based Composites Containing Multi-Walled Carbon Nanotubes for Thermal Energy Storage (TES) Development. Crystals 11(8): 951.
- Moura R, Seolatto A, De Oliveira Ferreira M, Freitas F (2018) The adsorption study of Royal Blue Tiafix and Black Tiassolan dyes using bone char as adsorbent. Adsorpt Sci Technol 36(3-4): 1178-1198.
- Cite P (2019) The development of bone char-based filters for the removal of fluoride from drinking water The development of bone char based filters.
- Brunson L, Sabatini D (2009) Sustainable use and implementation of bone char as a technology for ar-senic and fluoride removal. Water, Sanit Hyg Sustain Dev Multisectoral Approaches - Proc. 34th WEDC Int. Conf.

 Research Article
Research Article