ABSTRACT
Diabetes mellitus is a major health problem. It is characterized by increased concentration of blood glucose due to derangement in carbohydrates metabolism and defective insulin production. These metabolic disturbances result in acute and longterm diabetic complications. Most of the plants contain substances like glycosides, alkaloids, terpenoids, flavonoids etc and they are considered to be safe and effective for the treatment of diabetes. An attempt has been made to formulate silver nanoparticles with the herbal drug and evaluated against streptozotocin and nicotinamide induced type 2 diabetes in Wistar rats. Also, this research work focused on green chemistry which is an eco-friendly & environmentally accepted procedure. Pterocarpus marsupium silver nanoparticles are effective against an antidiabetic activity without using any chemical reagent as stabilizing agent. The in vitro and in vivo anti-diabetic study confirms that Pterocarpus marsupium is having anti-diabetic activity. The results suggested that Pterocarpus marsupium silver nanoparticle has more favorable reduction in lipid level in STZ and nicotinamide - induced diabetic rats, compared with glibenclamide as well as regeneration of ß-cells of pancreas. In conclusion it maybe stated that, there occurs a significant (P<0.01) decrease in the hyperglycemic state after the administration of Pterocarpus marsupium silver nanoparticle which reduce the severity of oxidative and acuity of hyperglycemia, a process that closely linked to glucose oxidation and formation of free radicals.
Keywords: Pterocarpus Marsupium; In vitro Study; In Vivo Study; Green Synthesis; Silver Nanoparticle; Anti- Diabetic
Introduction
Nanomaterials can be well-defined as a material with sizes ranged between 1 and 1000 nm, which influences the frontiers of nanomedicine starting from biosensors, microfluidics, drug delivery, and microarray tests to tissue engineering. Nanomedicines have become well appreciated in recent times due to the fact that nanostructures could be utilized as delivery agents by encapsulating drugs or attaching therapeutic drugs and deliver them to target tissues more precisely with a controlled release. Being nanosized, these structures penetrate in the tissue system, facilitate easy uptake of the drug by cells, permit an efficient drug delivery, and ensure action at the targeted location. The uptake of nanostructures by cells is much higher than that of large particles with size ranging between 1 and 10 μm. Hence, they directly interact to treat the diseased cells with improved efficiency and reduced or negligible side effects. Among the wide range of metal nanoparticles, silver nanoparticles (Ag-NPs or nano silver) were the most popular, due to their unique physical, chemical, and biological properties when compared to their macro scaled counterparts. In order to fulfill the requirement of AgNPs, various methods have been adopted for synthesis.
Generally, conventional physical and chemical methods seem to be very expensive and hazardous. Interestingly, biologically prepared AgNPs show high yield, solubility, and high stability. Several studies reported the synthesis of AgNPs using green, cost effective and biocompatible methods without the use of toxic chemicals in biological methods. The biological synthesis of nanoparticles depends on three factors such as the solvent, the reducing agent, the non-toxic material. Diabetes mellitus refers to the group of diseases that lead to high blood glucose levels due to defects in either insulin secretion or insulin action. Diabetes develops due to a diminished production of insulin (type 1) or resistance to its effects (in type 2) both of which leads to hyperglycemia. When the renal threshold for glucose reabsorption exceeds, glucose spill over into urine(glucosuria) and causes an osmotic diuresis (polyuria), which in turn results in dehydration, thirst and increased drinking (polydipsia). All forms of diabetes are treatable since insulin became medically available in 1921, but there is no cure. The injections by a syringe, insulin pump or insulin pen which is the basic treatment of type 1 diabetes. Type 2 is managed with combination of dietary treatment, exercise, medications and insulin. About 463 million people worldwide have diabetes, it is predicted that by 2045 it may afflict up to 700 million people. In India, currently 77 million individuals have diabetes, and it was expected to double in 2045.
Pterocarpus marsupium is a medicinal plant widely distributed in India. Pterocarpus marsupium is also known as Indian kino, Bijasal, Vijaysar, belongs to the family Fabaceae. Pterocarpus marsupium has been traditionally used in the treatment of leukoderma, elephantiasis, diarrhea, cough, discoloration of hair and rectalgia. It is nontoxic and useful in jaundice, fever, wounds, diabetes. The various parts used are bark, heartwood, bark, stem, leaves etc. Bark and wood have been used to treat diabetics and Pterostilbene significantly lower the blood glucose levels useful in NIDDM. In this study we synthesized silver nanoparticles using bark and wood extract of Pterocarpus marsupium and its characterization is done along with its in vitro and in vivo anti-diabetic study.
Materials and Methods
Reagents & Instruments Used for In Vitro Studies
Chemical Reagents: Silver nitrate, sodium potassium tartrate, α amylase enzyme, starch, 3,5- Dinitro salicylic acid, Sodium hydroxide, Sodium chloride, Sodium phosphate monobasic.
Equipment’s Used: Magnetic Stirrer, UV Spectrophotometer, FT-IR Spectrometer, pH meter, Zeta Sizer, SEM, Dialysis membrane 50.
Reagents & Instruments Used for In Vivo Studies
Chemicals Used: Ethyl acetate, Glucose, streptozotocin, nicotinamide, glibenclamide, potassium carbonate, ethanol, bovine serum albumin, sodium carbonate, sodium bicarbonate, potassium dihydrogen phosphate, sodium hydroxide, thiobarbituric acid, trichloro acetic acid, potassium dichromate, NADPH, glutathione, tris-HCl buffer, sodium azide, ellman’s reagent, hydrogen peroxide and glacial acetic acid were procured from sigma Aldrich.
Instruments Used: Centrifuge (Remi instruments Ltd., Kolkata), digital balance (Sartorius Ltd., USA), Shimadzu-Jasco V-630 UV/Vis’s spectrophotometer, ELECO 1/27 pH meter.
Procedure
Sample Collection
Pterocarpus marsupium bark were collected from Thalasseri, Kerala.
Authentication
The plant specimen was identified and authenticated by Botanical survey of India, Southern regional center, Coimbatore.
Drying and Pulverizing
The bark and wood were collected, and shade dried. It was grounded into fine coarse powder with electronic blender and passed and kept in a well closed container in a dry place.
Preparation of Aqueous Extract of Pterocarpus Marsupium Roxb
Fifty grams of the bark powder was stirred with 500 mL of deionized water and kept at 65 °C for 30mins. Then the extracts were filtered by using Whatman No. 1 filter paper after cooling to room temperature. The extract was stored at 4 °C for future use (Chen Yu, et al. [1]).
Phytochemical Screening
Preparation of Test Solution: The filtered aqueous extract of bark of Pterocarpus marsupium Roxb was used as a test solution for preliminary screening of phytochemical constituent.
Preliminary Qualitative Phytochemical Analysis
Phytochemical screening Chemical tests were carried out using the extract of Pterocarpus marsupium for the presence of phytochemical constituents.
Tests for tannins and phenolics
To the solution of the extract, a few drops of 0.1% ferric chloride was added and observed for brownish green or a blueblack coloration.
Tests for saponins
About 10ml of the extract was mixed with 5ml of distilled water and shaken vigorously for a stable persistent froth. The frothing was mixed with 3 drops of olive oil and shaken vigorously and then observed for the formation of emulsion.
Tests for terpenoids
About 5ml of the extract was treated with 2ml of chloroform and about 3ml concentrated H2SO4 was carefully added to form a layer. A reddish-brown coloration of the interface indicates the presence of terpenoids.
Tests for alkaloids
• A small portion of the extract was stirred with few drops of dil. HCl and filtered.
• To the filtrate, dragendroff’s reagent (potassium bismuth iodide solution) was added and an orange, brown precipitate indicates the presence of alkaloids.
• To the filtrate, Mayer’s reagent was added, and a cream precipitate indicates the presence of alkaloids.
Test for Starch
To the aqueous extract add weak aqueous Iodine solution.
Test for Proteins
Warming Test: Heat the test solution in a boiling water bath.
Test for Steroids
Salkowski Test: Treat the extract with few drops of concentrated sulphuric acid.
Preformulation Study
Solubility Test: About 1 mg of Pterocarpus marsupium Roxb. bark extract powder was taken in a test tube and solubility in ethanol, water, chloroform and diethyl ether, dimethyl sulphoxide were checked.
UV- VIS Spectral Analysis of Pterocarpus Marsupium Roxb Bark:
Preparation of Calibration Curve of Pterocarpus Marsupium Roxb Bark: Extract Twenty-five milligrams of crude extract were dissolved in phosphate buffer with a pH 7.4 and further diluted to 50mL of solvent, in volumetric flask to get a concentration of 500 μg /mL. This was treated as stock solution. Various aliquots of stock solution were diluted further to get different concentrations. The resultant solutions were scanned for λ max in the range of 200- 400nm using UV-spectrometer (Xi-Feng Zhang, et al. [2]).
FTIR Spectroscopy of Pterocarpus Marsupium Roxb Bark: 50 mg each of dried Pterocarpus marsupium Roxb bark and wood were mixed with 100 mg of spectral grade KBr and pressed into disc under hydraulic pressure. Then FTIR spectra were recorded in the 4000- 400cm-1 range (Holler, et al. [3]).
FTIR Spectroscopy of Silver Nitrate: 100mg of silver nitrate was mixed with 100 mg of spectral grade KBr and pressed into disc under hydraulic pressure. Then FTIR spectra were recorded in the 4000- 400cm-1 range (Holler, et al. [3]).
Green Synthesis of Silver Nanoparticles:
Preparation of Stock Solution: 1 mg of aqueous extract was weighed and diluted to 10 ml with distilled water.
Preparation of 1mm Silver Nitrate Aqueous Solution: 0.017g of silver nitrate was dissolved in 100 ml of distilled water to prepare 1mM solution of silver nitrate and stored in amber colored bottle until further use.
Synthesis of Silver Nanoparticles: An aliquot (1ml, 2ml,3ml, 4ml, 5ml) of aqueous plant extract sample was separately added to 10ml of 1mM aqueous AgNO3. To drive nanoparticle formation the reaction mixtures were kept in magnetic stirrer with constant stirring at 120 rpm. Color change of the reaction mixtures were monitored to determine silver nanoparticle formation which is indicated by a colloidal brown color (Shakeel Ahmed, et al. [4]).
Purification of AgNPs: The purification of AgNPs from the final reaction mixture. The reaction mixture (10ml AgNO3 + 5ml leaf extract sample) was split into two equal parts and transferred to pre-weighed sterile 15ml centrifuge tubes. The preparations were then centrifuged at 4000rpm for 20 mins. Supernatants were discarded and the pellets were collected & stored. The pellets were washed in 10ml of distilled water to remove any contaminating plant material before centrifugation for 1hr. This wash step was repeated twice to remove water soluble biomolecules such as proteins and cellular metabolites & then dried in an oven at 37 °C for 1 hr (Jerushka S Moodley, et al. [5]).
Characterization of Silver Nanoparticles: The present study includes time dependent formation of silver nanoparticles employing UV–Vis’s spectrophotometer, shape by employing FESEM and understanding of Pterocarpus marsupium silver nanoparticles interaction from Fourier transform infrared (FT-IR) spectroscopy, particle size measurement, stability from zeta potential and drug entrapment & Energy dispersive x-ray spectroscopy (EDS) for elemental analysis.
• UV- vis spectroscopy
UV-vis spectroscopy is a very useful and reliable technique for the primary characterization of synthesized nanoparticles which is also used to monitor the synthesis and stability of AgNPs. AgNPs have unique optical properties which make them strongly interact with specific wavelengths of light. In AgNPs, the conduction band and valence band lie very close to each other in which electrons move freely. These free electrons give rise to a surface plasmon resonance (SPR) absorption band, occurring due to the collective oscillation of electrons of silver nano particles in resonance with the light wave.
• Fourier transform infrared spectroscopy
FTIR is able to provide accuracy, reproducibility, and also a favorable signal-to-noise ratio. FTIR is a suitable, valuable, noninvasive, cost effective, and simple technique to identify the role of biological molecules in the reduction of silver nitrate to silver.
• Field emission scanning electron microscopy
Among various electron microscopy techniques, SEM is a surface imaging method, fully capable of resolving different particle sizes, size distributions, nanomaterial shapes, and the surface morphology of the synthesized particles at the micro and nanoscales. Using SEM, we can probe the morphology of particles and derive a histogram from the images by either by measuring and counting the particles manually, or by using specific software.
• Energy dispersive x-ray spectroscopy
The combination of SEM with energy-dispersive X-ray spectroscopy (EDX) can be used to examine silver powder morphology and also to conduct chemical composition analysis.
• Determination of Zeta potential
Zeta potential is a measure of surface charge. The surface charge (electrophoretic mobility) of nanoparticles can be determined by using Zeta sizer (Malvern Instrument) having zeta cells, polycarbonate cell with gold plated electrodes and using water as medium for sample Preparation. It is essential for the characterization of stability of the silver nanoparticles.
• Particle Size Determination
The average mean diameter and size distribution of silver nanoparticles is found by Dynamic Light Scattering method using Malvern zeta sizer at 25°C.The dried nanoparticles were dispersed in water to obtain proper light scattering intensity for silver nanoparticles.
• Determination of Entrapment Efficiency
The entrapment efficiency of nanoparticles was determined by adding 10 ml of phosphate buffer of pH 7.4 and sonicated in a bath sonicator and filtered. 1 ml of filtrate is made up to 10 ml with phosphate buffer and was assayed spectrophotometrically at 337 nm (UV visible spectrophotometer, JASCO V-530. The amount of entrapped drug was calculated from the equation. (www.scieconf. com) [6].
In-vitro Methods
α- Amylase Inhibitory Effect: Pancreatic α-amylase, an important enzyme of digestive system hydrolyzes starch into mixture of smaller oligosaccharides comprising of maltose, maltotriose and oligoglucans which are further degraded by glucosidase into glucose that enters the blood stream upon absorption. This leads to elevated post-prandial hyperglycemia (PPHG). Hence, it is important to control these two aspects in the treatment of type 2 diabetes.
Procedure: From 1mg/ml stock solution different concentrations of plant extracts were prepared in phosphate buffer. About 500μl of test/standard was added to 500 μl of α-amylase (0.5mg/ml) is incubated for 10min at room temperature. Then added 500μl of 1% starch solution and incubated for another 10minutes. After that 1ml of coloring reagent was added to reaction mixture it is prepared by mixing sodium potassium tartrate solution (12g dissolved in 8ml of 2M NaOH) and 96Mm 3,5- Dinitro salicylic acid and heated in boiling water bath for 15minutes after cooling, 10ml of distilled water is added. To measure the absorbance of colored extracts blank is prepared for each set of concentration of test sample by replacing the enzyme with buffer. Control incubations representing 100% enzyme activity was prepared by replacing test drug with buffer. Absorbance measured at 540 nm.
The positive control used for this assay is acarbose which works by slowing the action of certain chemicals that break down food to release glucose into your blood. Slowing food digestion helps keep blood glucose from rising very high to after meals (Vishnu Kiran M, et al. [7]).
In Vitro Drug Release Study: The antidiabetic drug Pterocarpus marsupium loaded silver nanoparticles (300 mg) were suspended in 10 mL of phosphate-buffered saline (PBS) in a dialysis bag. The dialysis bag was sealed and then slowly shaken in 90 mL of PBS at 37°C in a 250-mL beaker and kept in a magnetic stirrer at an rpm 170. Aliquots of the solution outside the dialysis membrane (2 mL) were replaced with 2 mL of PBS at various times intervals and tested at 427 nm by UV Spectrophotometer. The change of the concentrations of drug with respect to different time intervals were obtained from curves of the absorbance A versus concentration C of Pterocarpus marsupium silver nanoparticles in PBS based on Lambert-Beer law (Guo-Ping Yan, et al. [8]).
Experimental Animals: Female Wistar rats weighing 180- 200 g were used for acute toxicity and antidiabetic activity studies. The animals were kept in polypropylene cages under ambient temperature (22 ± 3 ᴼC) with 12 hrs light/dark cycle. Animals were provided with standard pellet feed and drinking water ad libitum. All animal procedures were performed in accordance with the recommendation for the proper care and use of laboratory animals.
Acute Toxicity Study: Acute oral toxicity testing was carried out in accordance with the OECD guidelines 420 Acute Oral Toxicity – Fixed Dose Procedure method (OECD, 2002).
Procedure: Healthy adult female (generally slightly more sensitive than male) Wistar rats weighing between 180-200 g body weight were procured from Kerala veterinary animal sciences university, Kerala and kept in cages under ambient temperature (22 ± 3 ᴼC) with 12 hrs light/dark cycle. The animals were randomly selected, marked and kept in their cages for 7 days prior to dosing for acclimatization to laboratory conditions. The animals were fasted overnight and were provided with water ad libitum. The test compounds were suspended in 0.5% CMC. Totally 9 animals were used for this study. Sighting study was conducted with 2000 mg/kg body weight. The animals survived without any toxic manifestations during the sighting study and the same dose was selected for the main study. The main study was conducted for a drug at dose of 2000 mg/kg body weight using 8 animals. After the administration of the drug, food was withheld for further 3-4 hours. Animals were observed individually at least once during the first 30 min after dosing, periodically during the first 24 hrs (with special attention during the first 4 hrs.) and daily thereafter for a period of 14 days. Once daily cage side observations included changes in skin and fur, eyes and mucous membrane (nasal), and also respiratory rate, circulatory (heart rate and blood pressure), autonomic (salivation, lacrimation, perspiration, piloerection, urinary incontinence and defecation) and central nervous system (drowsiness, gait, tremors and convulsions) changes.
Experimental induction of diabetes was induced by intraperitoneal injection of streptozotocin (60 mg/kg) dissolved in 0.1M cold sodium citrate buffer (pH 4.5) in overnight fasting rats. Nicotinamide 120 mg/kg was administered 15 minutes prior to the administration of streptozotocin to all animals except control group. The control rats received vehicle alone, and all animals in group 2 to group 5 were allowed to drink 5% glucose solution overnight to overcome the drug-induced hypoglycemia. After 1week time, the rats with moderate diabetes having glycosuria and hyperglycemia (blood glucose range of above 250 mg/dl) were considered as diabetic rats and used for the experiment (Anoop S, et al. [9]).
Experimental Design: The rats were divided into 5 groups, consisting of 8 animals in each group.
• Group 1: Control rats, received saline 10 ml/kg
• Group 2: Diabetic control rats, received Streptozotocin 60 mg/ kg + Nicotinamide 120mg/kg i.p
• Group 3: Diabetic rats received 2.5 mg/kg, Glibenclamide p. o.
• Group 4: Diabetic rats, received 200 mg/kg, Pterocarpus marsupium p. o.
• Group 5: Diabetic rats, received 200 mg/kg, Pterocarpus marsupium silver nanoparticles p. o.
Glibenclamide (2.5 mg/kg) used as standard drug. All the test drugs were administered orally and treatment was continued for 28 days.
Sample Collection: Blood samples were collected from tip of rat tail and blood glucose levels were estimated using glucocheck electronic glucometer weekly basis (0, 7 and 14 days) and body weight measured at weekly intervals before blood glucose estimation.
Estimation: On 15th day of experiment blood was collected from the retro-orbital plexus using ketamine/xylazine anesthesia using capillary tubes in fresh vials containing EDTA and serum was separated. Serum analyzed for total protein and the values were tabulated. Rats were sacrificed by cervical dislocation under ketamine/xylazine anesthesia on 15th day and liver tissue was removed and used for the preparation of homogenates for biochemical estimation.
Tissue Processing of Liver: The tissue was removed and washed with ice-cold saline to remove as much as blood possible. Liver was homogenated (5%w/v) in cold potassium phosphate buffer (50Mm, Ph 7.4) using a Remi homogenizer. The unbroken cell and cell debris were removed by centrifugation at 3000rpm for 10min. The obtained supernatant was used for the estimation of total protein, malondialdehyde, superoxide dismutase, catalase, glutathione reductase, glutathione peroxidase and reduced glutathione.
Statistical Analysis: Statistical analysis was performed using one way ANOVA followed by Dunnett’s test. Values are expressed as mean ±SEM and p<0.05 were considered statistically significant.
Results & Discussion
Preformulation Studies
Physical Characteristics: Pterocarpus marsupium was checked for its color, odor and texture. It is light yellow colored powder in appearance and has a pleasant odor.
Phytochemical Screening: Pterocarpus marsupium was an excellent bio-source of alkaloids, glycosides, carbohydrates and flavonoids, whereas tannins and phenols, saponins test were found to be negative. These are the results for the screening of phytoconstituents present in Pterocarpus marsupium. Pterostilbene is the flavonoid present in the bark of Pterocarpus marsupium which is responsible for the antidiabetic activity (Treas and Evans [10]).
Solubility Studies: From the solubility studies, it has cleared that the Pterocarpus marsupium bark extract is soluble in water, ethanol & phosphate buffer 7.4 and sparingly soluble in chloroform & dimethyl sulphoxide. Hence Pterocarpus marsupium aqueous extract of bark is more soluble in polar solvents than non-polar solvents.
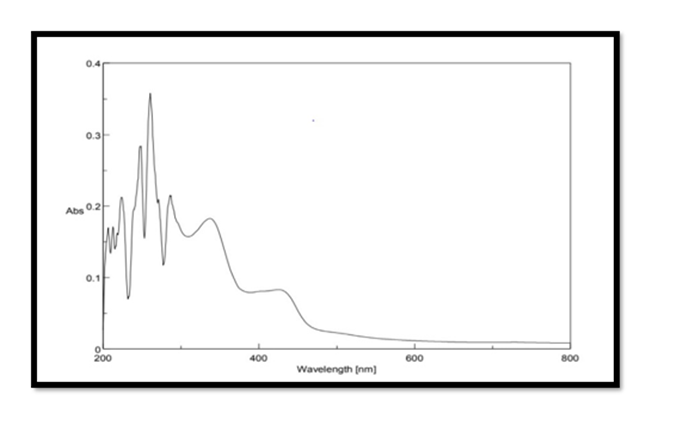
Selection of wavelength: The Pterocarpus marsupium stock solution of concentration 500μg/mL was scanned in the range of 200 – 800 nm for λmax using double beam UV Spectrophotometer. The maximum absorption of Pterocarpus marsupium was found to be at 337nm and hence it is selected as the wavelength for further studies.
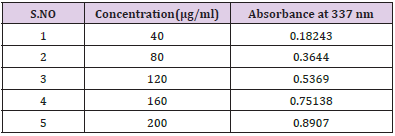
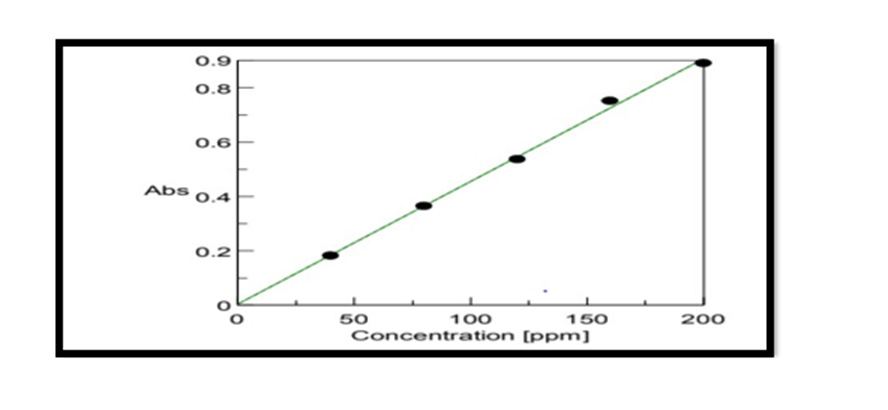
Construction Of Calibration Curve of Pterocarpus Marsupium Bark: To construct a calibration curve, 40 – 200 μg/ml of Pterocarpus marsupium was taken & checked the linearity at 337 nm. The calibration data is shown in the (Table 1). In the calibration curve, linearity was obtained between 40-200μg/ml concentration of Pterocarpus marsupium bark and the regression value was found to be r2 = 0.99837. Hence, we can conclude that Pterocarpus marsupium bark obeys Beer Lambert’s Law at the concentration between 40-200 μg/ml Figure 1.
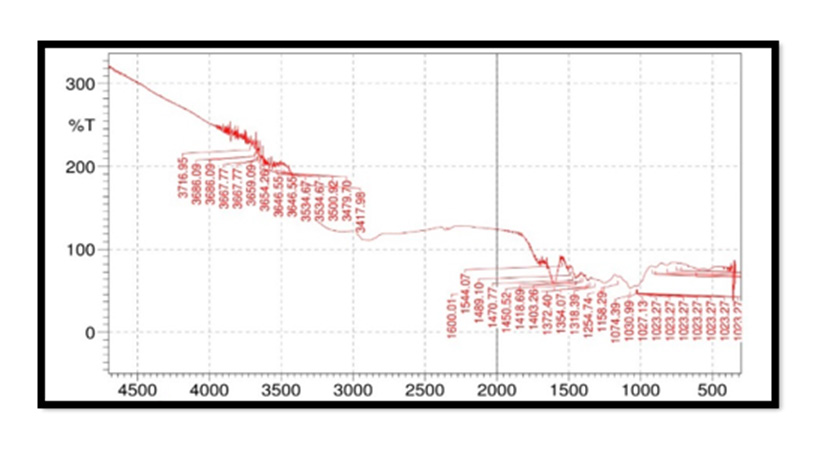
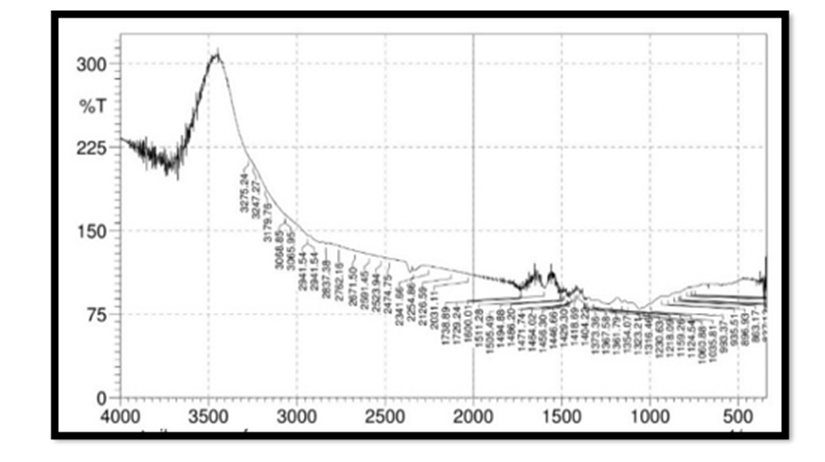
FTIR Spectroscopy of Pterocarpus Marsupium: Fourier Transform Infrared (FT-IR) spectra of the samples were obtained using a FTIR Jasco 4100 Spectrometer by KBr disc method. The spectrums were recorded for the pure drug and physical mixture of drug and excipient and are shown in Figure 2. In the Pterocarpus marsupium bark FTIR spectrum strong absorption peaks at 3500.92 and 3534.67 indicates OH stretching due to the presence of alcohol and phenol Figure 3.
Characterization
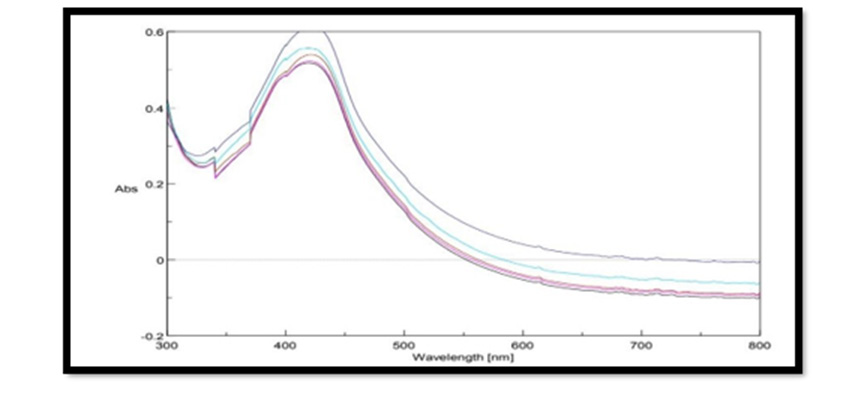
UV Vis Spectral Analysis of Pterocarpus Marsupium Silver Nanoparticles: Periodic sampling of 30 mins, 60 mins, 90 mins, 210 mins & 24 hrs were taken by using distilled water as blank from the wavelength of 300 – 800 m which is depicted in the Figure 4. In AgNPs, the conduction band and valence band lie very close to each other in which electrons move freely. These electrons give rise to a surface plasmon resonance absorption band in the visible region occurring due to the collective oscillation of electrons of silver nanoparticles in resonance with the light wave. The silver surface plasmon resonance was observed at 427nm which steadily increases in intensity as a function of time of reaction (ranging from 30 min to 5 h) without showing any shift of the wavelength maximum (Xi-Feng Zhang, et al. [2]) Figure 5.
Figure 5: Uv- visible absorption spectra of pterocarpus marsupium Roxb. silver nanoparticles at different time intervals.
FTIR Spectroscopy of Pterocarpus Marsupium Silver Nanoparticles: In the FTIR spectra of silver nanoparticles band between 3275.24-3247.27 corresponds to OH stretching of free alcohol, 3179-2941 corresponds to CH stretching in aldehyde, 1060- 1464 C=O stretching in carboxylic acid, 1354-1404 corresponds to nitro compounds.
Drug Entrapment Efficiency: The drug entrapment can be calculated after centrifugation by UV spectrophotometry at 427 nm. The amount of drug present in the supernatant liquid was calculated by using the following formula.
% Drug entrapment = W − w / W * 100
W – total amount used in the preparation of silver nanoparticles
w- drug present in the supernatant obtained from calibration curve
(W-w) – amount of drug entrapped (Peng-Fei Yue [11]).
The % entrapment of drug or drug content of Pterocarpus marsupium Roxb. silver nanoparticles were found to be 81% for F1, 83% for F2, 85% for F3, 89% for F4 and 93% for F5. Hence the highest amount of drug was entrapped in the formulation F5 which is 93% also it was in colloidal brown color indicated the formation of Pterocarpus marsupium silver nanoparticles. From the drug entrapment results, the formulation F5 was chosen for further evaluation studies because it possessed high drug entrapment than the other formulations [12].
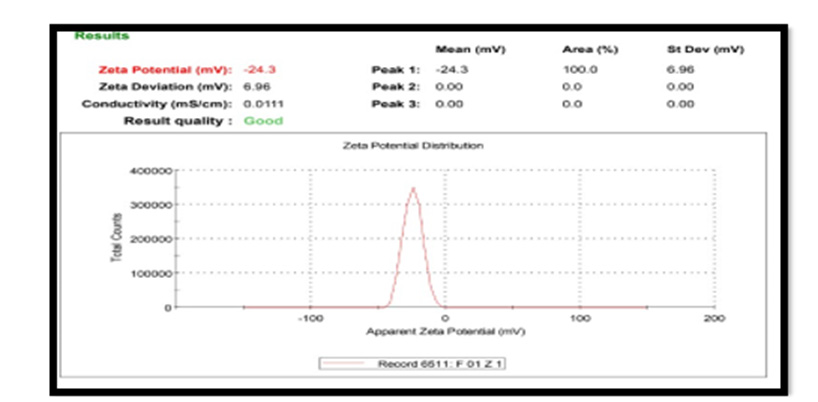
Zeta Potential: For Pterocarpus marsupium silver nanoparticles zeta potential was found to be -24.3 mV with peak area 100 intensity. These values indicate that the formulated Pterocarpus marsupium silver nanoparticles are stable. Zeta potential distribution of silver nanoparticles are depicted in the Figure 6. Nanoparticles with zeta potential value greater than +25 mV or less than - 25 mV typically have high degrees of stability. If all the particles in suspension have a large negative or positive zeta potential, then they will tend to repel each other and there will be no tendency for the particles to come together. However, if the particles have low zeta potential values, then there will be no force to prevent the particles coming together and flocculating [13-20].
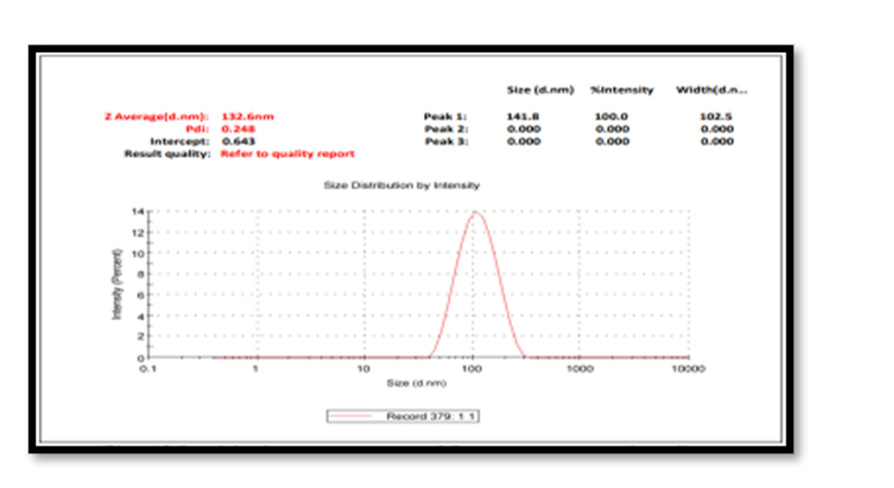
Particle Size Measurement: The average particle size (z-average) is found to be 132.6 nm. Particle size analysis showed the presence of nanoparticles with polydispersity indices PDI value 0.248 with intercept 0. 643. The colloidal solution having a Poly Dispersity Index less than 0.50 are considered as good quality. The results were in agreement with the statement reported before. Physical properties vary greatly with size change in the nanoscale, melting points drop dramatically with smaller nano size; optical absorption is also sensitive to size, where Ag exhibit plasmon absorptions in the 400–600 nm range wavelengths depending on size [21-25] Figure 7.
Field Emission Scanning Electron Microscopy: FE SEM analyses of the formulated Pterocarpus marsupium silver nanoparticles were performed to evaluate the surface morphology of nanoparticles. The FESEM image of Pterocarpus marsupium silver nanoparticles displayed below, reveals that the particles were Uniform in size, Spherical in shape and segregated. The FESEM image of silver nanoparticles synthesized by green synthesis process by using 5 % bark extract and 1mM AgNO3 concentration gave a clear image of highly dense silver nanoparticles Figures 8 & 9. The FESEM image showing silver nanoparticles synthesized using Pterocarpus marsupium aqueous extract of bark confirmed the growth of silver nanostructures [25-30].
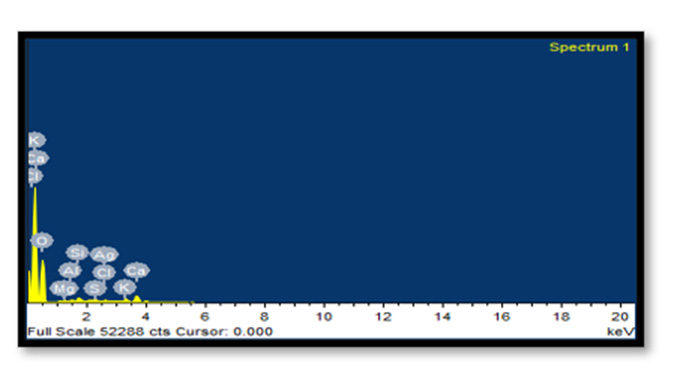
Energy Dispersive X-Ray Spectroscopy: EDX analysis of AgNPs demonstrated a well-defined silver signal at 3 keV along with carbon, oxygen and nitrogen peaks, with the latter weaker signals probably representing surface biomolecule capping structures originating from the bark extracts.
In Vitro Antidiabetic Study
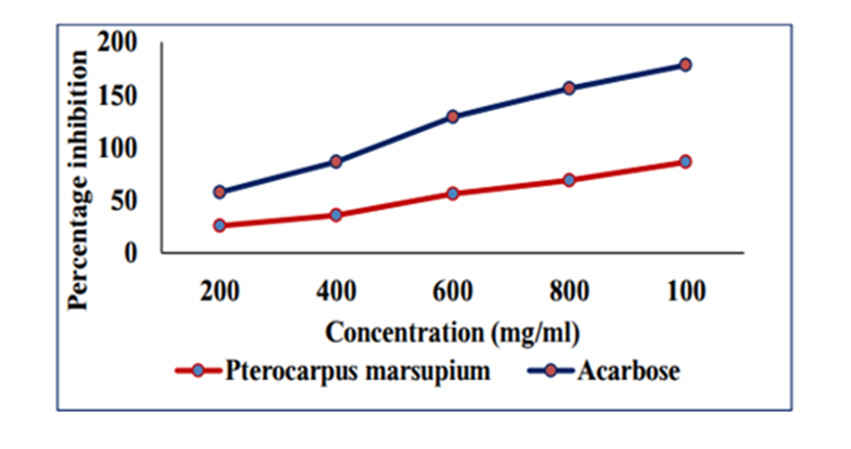
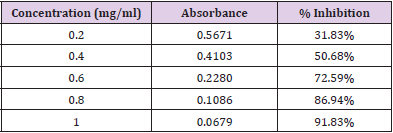
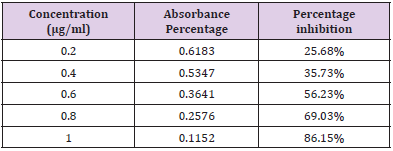
In Vitro Alpha Amylase Inhibition: α-amylase is a key enzyme in carbohydrate metabolism. Inhibition of α-amylase is one of the strategies of treating diabetes. Inhibiting α-amylase will lower post prandial blood sugar. The result suggest that Pterocarpus marsupium silver nanoparticles exhibit good α amylase activity under in vitro condition. Dose dependent % inhibitory activity against α-amylase was noted. Our study indicates that Pterocarpus marsupium could be useful in the treatment of post prandial hyperglycemia. The anti-diabetic activity may be attributed to the presence of flavonoids, tannins & anti α-amylase activity. Acarbose is used as a standard here which is a good antidiabetic drug and works by slowing the action of certain chemicals that breakdown food to release glucose into our blood. The absorbance of control without sample is taken and it is 0.832. Percentage inhibition of α amylase for the positive control Acarbose was found to be 31.83% at concentration 0.2mg/ml. Percentage inhibition of α amylase for the pterocarpus marsupium Roxb. silver nanoparticles were found to be 25.68% for the concentration 0.2mg/ml [31-40]. The percentage α amylase inhibition of positive control Acarbose at lower(0.2mg/ ml) and higher (1mg/ml) concentration were found to be 31.83% and 91.83% and for test pterocarpus marsupium Roxb. silver nanoparticles percentage α amylase inhibition at lowest (0.2mg/ ml) and highest (1mg/ml) concentration were found to be 25.68% and 86.15% respectively [41-45] Figures 10 & 11.
Figure 11: amylase inhibition of Pterocarpus marsupium silver nanoparticles & acarbose on alpha amylase enzyme.
In Vivo Results
Acute Toxicity Studies and Selection of Dose for In-Vivo Studies
The aqueous extract of bark of Pterocarpus marsupium silver nanoparticles were selected and used for further in vivo evaluation. Acute toxicity was carried out as per OECD guidelines 420 employing fixed dose procedure for selecting the dose for biological activity. For acute toxicity studies female wistar rats weighing 180- 195gms were taken and they were fasted overnight before the experimental day. Overnight fasted rats were weighed, and body weight determined for dose calculation and test compound were administered orally. 2000 mg/kg dose of Pterocarpus marsupium silver nanoparticles and the rats were observed for signs of acute toxicity. No toxic effect was observed after sufficient interval of time (2-3days). Signs and symptoms of toxicity and death if any were observed individually for each rat at 0, 0.5, 1, 2, 3 and 4h for first 24h and thereafter daily for 14 days. Diet was given to the animals after 4th hour of dosing. The animals were observed twice daily for 14 days, and body weight changes, food and water consumption were noted. In acute toxicity studies, it was found that the animals were safe up to a maximum dose of 2000mg/kg of body weight (Tables 2 & 3). There were no changes in normal behavioral pattern and no signs and symptoms of toxicity and mortality in rats. As per the OECD 420 guidelines Pterocarpus marsupium silver nanoparticles can be included in the category 5 or unclassified category of globally harmonized classification system (GHS) [45-50]. Hence based on these results the Pterocarpus marsupium silver nanoparticles were considered non-toxic and 1/20th dose were used for the biological evaluation (antidiabetic activity) and the studies were conducted at dose levels of 2000 mg/kg body weight [51].
Table 3: α- Amylase inhibitory effects of Pterocarpus marsupium Roxb. silver nanoparticles. Comparison of percentage inhibition of both positive and test drug.
In Vivo Antidiabetic Activity of Pterocarpus Marsupium Silver Nanoparticles
The biological evaluation was carried out using 2000mg/ kg dose of aqueous bark extract of Pterocarpus marsupium silver nanoparticles. The body weight of control and experimental animals on 0, 7, 14, 21, 28 days of treatment. There was significant reduction of body weight in diabetic control animals compared to normal control & test drug treated animals. On 7th day significant reduction of body weight was observed in diabetic control animals (159.85±3.95) when compared to control rats (193.14±2.47). Reduction in body weight indicates the induction of diabetes. The normal control (209.28±2.28) and drug treated rats pterocarpus marsupium aqueous extract, Pterocarpus marsupium silver nanoparticles, glibenclamide gained significant weight (172.42±9.19, 175.57±3.32, and 173±8.3) (P<0.01) on 28 days of treatment. The effect of pterocarpus marsupium aqueous extract, Pterocarpus marsupium silver nanoparticles, glibenclamide on streptozotocin and nicotinamide induced diabetes in rats. Initially it was found that a significant (P<0.01) increase in blood glucose level was observed in STZ-nicotinamide induced diabetic rats (243±4.601) compared to normal control (80.5±3.12). After the daily treatment for 14 days showed significant (p<0.05), (P<0.01) reduction in blood glucose 2000 mg/kg p.o of aqueous extract of Pterocarpus marsupium (195±2.90), aqueous extract of Pterocarpus marsupium silver nanoparticles (179.25±1.493), 2.5 mg/kg, p.o of glibenclamide (165.25±3.78) as compared to diabetic control group. (243 ± 4.60) ##
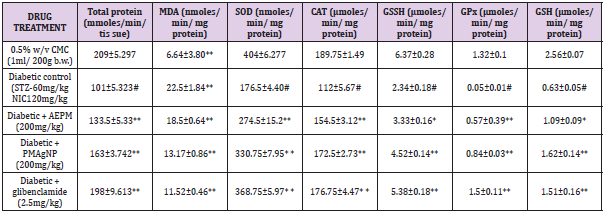
The level of protein after 28 days of treatment in liver is shows that decrease in protein level was found in diabetic control (101±5.323) rats compared with control rats (209±5.297). Administration of Pterocarpus marsupium aqueous extract, Pterocarpus marsupium silver nanoparticles (2000mg/kg), (133.5+5.33,163+3.742) and glibenclamide (2.5 mg/kg) treated rats (198.25±9.613) restored the protein level significantly near to normal levels. The results were found to be statistically significant (P<0.01). A significant (P<0.01) increase was observed in the activities of SOD in the treatment groups administered with, Pterocarpus marsupium aqueous extract, Pterocarpus marsupium silver nanoparticles (200 mg/ kg), glibenclamide (274.5±15.2, 330.75±7.95, 368.75 ± 5.97) CAT (154.5±3.12, 172.5±2.73, 176.75±4.47) GSSH(3.33±0.16, 4.52±0.14, 5.38±0.18) GPX (0.57±0.39, 0.84±0.003, 1.5±0.11) and GSH (1.09±0.09, 1.62±0.14, 1.51±0.16) in liver homogenates of diabetic rats compared to control rats. Administration of pterocarpus marsupium silver nanoparticles (2000mg/kg) (172.5±2.73) and standard glibenclamide (176.75±4.47) significantly (P<0.01) increased the activity of CAT enzymes compared to the negative control group (112±5.67). The level of GSSH (4.52±0.14, 3.33±0.16, 5.38±0.18) and GPX (0.57±0.39,0.84±0.03,1.5±0.11) level were also increased with the treatment of Pterocarpus marsupium aqueous extract Pterocarpus marsupium silver nanoparticles (2000 mg/kg), glibenclamide (2.5mg/kg) compared with the negative control in liver. (112±5.67) [52-57] (Table 4).
Table 4: Effect of Pterocarpus marsupium silver nanoparticles on the serum in in streptozotocin and nicotinamide-induced type 2 diabetes mellitus rats.
Note: Each value represents the mean ± SEM, n= 8 In negative group, # P< 0.01 Vs control Group IV & V ** P < 0.01 Vs Group II and Group III *P< 0.05 Vs group II.
Data were analyzed by one-way ANNOVA followed by Dunnet’s test.
The non-enzymatic antioxidant, GSH level also decreased in diabetic control group compared with the control group. Treatment with Pterocarpus marsupium aqueous extract 2000 mg/kg (1.09±0.09) pterocarpus marsupium silver nanoparticles 2000 mg/ kg (1.62±0.14) and glibenclamide (1.51±0.16) significantly (P<0.01) increases this enzymes level compared with the negative control group in liver. The effect of Pterocarpus marsupium bark extract & its silver nanoparticles on MDA shown in table. MDA level was found to be elevated in streptozotocin and nicotinamide-induced diabetic rat (22.5±1.84) compared to control rats (6.64±0.3.80) in liver. This level was significantly (P<0.01) reduced in the diabetic rats treated with the, Pterocarpus marsupium bark extract & its silver nanoparticles 2000 mg/kg (18.5±0.64, 13.17±0.86) and standard glibenclamide (11.52±0.46) treated groups. Streptozotocin (STZ) is 1-methyl-l-nitrosourea attached to the carbon-2 position of glucose that causes β-cell necrosis and induces experimental diabetes in many animal models. It causes DNA strand breaks that induce the activation of poly-ADP-ribose synthetase followed by lethal nicotinamide adenine dinucleotide (NAD) depletion. Nicotinamide adenine dinucleotide causes activation of the poly ADP ribose synthase to repair the damaged DNA and protecting the decrease in the level of NAD and proinsulin thereby partially reversing the inhibition of insulin secretion to prevent the aggravation of experimental diabetes. This condition shows a number of features which are similar with type 2 diabetic mellitus (T2DM). Hence, based on this point of view, the hypoglycemic activity of Pterocarpus marsupium silver nanoparticles carried out on STZ and nicotinamide induced type 2 diabetic rats.
In streptozotocin and nicotinamide-induced type 2 diabetic mellitus there was a significant reduction in body weight in diabetic rats is due to excessive break down of tissue protein. Treatment with Pterocarpus marsupium silver nanoparticles & glibenclamide improved body weight significantly inducing prevention of muscle wasting due to hyperglycemic condition. The difference in body weight is large in control group compared with negative control group. In treatment group also, there is an increase in the body weight compared with the diabetic control group. Generation of free radicals in diabetes mellitus reacts with lipids causing lipid peroxidation, resulting in the release of products such as malondialdehyde, hydroperoxide and hydroxyl radicals. The oxidative stress in diabetes decreases the antioxidant status. SOD, CAT, GSSH and GPx are enzymatic antioxidants and non-enzymatic antioxidant like GSH plays an important role in protecting cells from being exposed to oxidative damage by direct elimination of reactive oxygen species (ROS).
CAT and SOD are considered primary enzymes since they are involved in the direct elimination of ROS. SOD is an important defense enzyme which catalyzes the dismutation of superoxide radical and CAT is a hemoprotein which catalyzes the reduction of H2O and protects the tissue from hydroxyl radicals. GPX, a selenium containing enzyme present in significant concentration detoxifies H2O2 to H2O through the oxidation of reduced glutathione. The reduced activity of SOD, CAT, GPX, GSSH, GSH in the liver during diabetes is a result of deleterious effects which results in the accumulation of superoxide anion radicals and H2O2. The activity of enzymatic and non-enzymatic antioxidants is increased significantly in Pterocarpus marsupium silver nanoparticle treated animals (P<0.01). Marked increase in the concentration of MDA was observed in the liver of diabetes rats. Pterocarpus marsupium silver nanoparticle and glibenclamide tends to bring the increased concentration of lipid peroxidation products to near normal level.
Conclusion
In conclusion it maybe stated that, there occurs a significant (P<0.01) decrease in the hyperglycemic state after the administration of Pterocarpus marsupium silver nanoparticle which reduce the severity of oxidative and acuity of hyperglycemia, a process that closely linked to glucose oxidation and formation of free radicals. Our results suggested that Pterocarpus marsupium silver nanoparticle has more favorable reduction in lipid level in STZ and nicotinamide - induced diabetic rats, compared with glibenclamide as well as regeneration of ß- cells of pancreas. The present study suggests that Pterocarpus marsupium silver nanoparticles can be successfully utilized for the management of diabetes due to their anti-hyperglycemic action.
Acknowledgement
None.
Conflict of Interest
None.
Financial Support
None.
Ethics Statement
All procedures performed in studies involving animals were in accordance with the ethical standards of the institutional animal ethics committee at which the studies were conducted (Approval #: 1559/PO/Re/11/CPCSEA).
References
- Chen yu, Jingchun Tang, Xiaomei Liu, Xinwei Ren, Meinan Zhen, et al. (2018) Green Biosynthesis of Silver Nanoparticles Using Eriobotrya japonica (Thunb.) Leaf Extract for Reductive Catalysis 12(1): 189.
- Xi Feng, Zhang (2016) Silver Nanoparticles: Synthesis,’ Characterization, Properties, Applications, and therapeutic approaches. International journal of molecular science 17(9): 1534.
- Holler, Skoog, Crouch, Principles instrumental analysis, edition. 6: 459-461.
- Shakeel Ahmed (2016) A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. Journal of Advanced Research 7(1): 17 -28.
- Jerushka S Moodley, Suresh Babu Naidu Krishna, Karen Pillay, Sershen, Patrick Govender, et al. (2018) Green synthesis of silver nanoparticles from Moringa oleifera leaf extracts and its antimicrobial potential 9(1).
- scieconf.com
- Vishnu kiran M, Murugesan S (2017) Investigation of biosynthesized silver nanoparticles from interaction from Halymenia porphyroides with the E7 protein using bioinformatics tool. Biomedical journal of Science and Technical Research 1(4): 1-4.
- Guo ping yan, Syed Asad Hussain Bukhari, Jian-binZENG, Xiao-yanQu, ANEssaAli, et al. (2017) Nitrogen (N) metabolism related enzyme activities, cell ultrastructure and nutrient contents as affected by N level and barley genotype. Journal of Integrative Agriculture 16(1): 190-192.
- Anoop S, Firdous (2016) Effect of Ipomoea staphylina leaves on Streptozotocin - Nicotinamide Induced Type-II Diabetes in wistar Rats. Asian Pacific Journal of Health Sciences 3(3): 30-44.
- Evans W (2002) Trease and Evans pharmacognosy (15th)., Elsevier Ltd. Philadelphia, pp. 414-420.
- Peng Fei Yue, Xiu-Yun Lu, Zeng Zhu Zhang, Hai Long Yuan, Wei Feng Zhu, et al. (2009) The Study on the Entrapment Efficiency and In Vitro Release of Puerarin Submicron Emulsion. American Association of Pharmaceutical Science 10(2): 376-383.
- Abdulfatai B (2012) Type 2 diabetes mellitus: A Review of current trends. Oman Medical Journal 27(4): 269-273.
- Aguilar Bryan L, C G Nichols, S W Wechsler, J P Clement, A E Boyd, et al. (1995) Cloning of the beta cell high-affinity sulfonylurea receptor: A regulator of insulin secretion. Science 268(5209): 423-426.
- (1997) American diabetes association. Report of the expert committee on the diagnosis and classification of Diabetes Mellitus. Diabetes Care 20(7): 1183-1197.
- (2019) American diabetes association. Classification and diagnosis of diabetes. Diabetes care 42(1): 13 -28.
- Bagya Lakshmi J, Haritha (2017) Green Synthesis and Characterization of Silver Nanoparticles Using Pterocarpus marsupium and Assessment of its In vitro Antidiabetic Activity. American Journal of Advanced Drug Delivery 5(3).
- Baynes HW (2015) Classification, pathophysiology, diagnosis and management of diabetes. Journal of Diabetes and Metabolism 6(5): 541.
- Bhor VM, Raghuram N, Sivakami S (2004) Oxidative damage and altered antioxidant enzyme activities in the small intestine of streptozotocin induced diabetic rats. International Journal of Biochemistry and Cell Biology 36(1): 89-97.
- Birangane R, Chole D, Sathya P (2011) A Review of antioxidants. Journal of Indian Academy of Oral Medicine and Radiology 23(5): 351-353.
- Bonaventura C (2015) Gestational diabetes mellitus: Challenges in diagnosis and management. Journal of diabetes metabolic disorder 14(3): 42-52.
- Bruce BL, G A Spiller, L M Klevay, S K Gallagher (2000) A diet high in whole and unrefined foods favorably alters lipids, antioxidant defenses, and colon function. Journal of the American College of Nutrition. 19(1): 61-67.
- Dsouza D, Lakshmidevi N (2015) Models to study In vitro Antidiabetic activity of plants. International Journal of Pharma and Bio Sciences 6(3):732-741.
- Ibrahim khan (2019) Nanoparticles: Properties, applications and toxicities. 12(7): 908- 931.
- Jacob V, Michel A (1999) Nutrtional Antioxidants: Mechanism of action, analysis of activities and medical application. Nutrition 49(21): 1-7.
- Johnas S (2018) In vitro antidiabetic activity and mechanism of action of Brachylaena elliptica. Journal of Evidence Based Complementary and Alteration Medicine 10(1): 113.
- Kakkar P, Das B (1984) A modified spectrophotometric assay of SOD. Indian Journal of Biochemistry and Biophysics 2(1): 130-132.
- Kirtikar KR, Basu BD (1935) In: Indian Medicinal Plants -Vol 3 (2nd )., Dehradun, pp. 1647-1648.
- Kishore L, Kajal A (2017) Role of Nicotinamide in Streptozotocin Induced Diabetes in Animal Models. Journal of Endocrinol thyroid research 2(1): 1-4.
- Kokate C K, AP Purohit, SB Gokhale. Pharmacognosy. volume 1 & 2 (45th)., p. A.1 to A.6.
- Krishna A (2011) Different techniques used for the preparation of nanoparticles using natural polymers and their applications. International Journal of Pharmaceutical Sciences. 3(2): 45-50.
- Lowry OH, N J Rosebrough, A L Farr, R J Randall (1951) Protein measurement with the folin phenol reagent. Journal of Biological Chemistry 193(1): 265-275.
- Manisha M, Priyanjali Dixit, Jayant Londhe, Saroj Ghaskadbi, Thomas Paul A Devasagayam, et al. (2007) Indian herbs and herbal drugs used for the treatment of diabetes. Journal of Clinical Biochemistry and Nutrition 40(3): 163-173.
- Mayfield J (1998) Diagnosis and classification of diabetes mellitus; new criteria. American Family Physician 58(6): 1355-1362.
- Meena shamrao deogade (2016) Pharmacognostical and Phytochemical study of costus igneus NE Br leaf. Journal of Indian System of Medicine 2(4): 174-178.
- Mohd saidur Rahman, Mujahid, Mohd Aftab Siddiqui, Azizur Rahman, Muhammad Arif, et al. (2018) Ethnobotanical Uses, Phytochemistry and Pharmacological Activities of Pterocarpus marsupium: A Review 10(6): 1-8.
- Nathaniel (2007) Symptoms of diabetes and their association with the risk and presence of diabetes 30(11): 2868-2873.
- Neelu Chauhan (2018) Silver nanoparticles: Synthesis, Characterization and Applications. p. 4- 9.
- Neeti S (2014) Free radicals, antioxidants and disease. Biology and medicine 6(3): 2-6.
- Nidhi Mishra (2016) Targeted drug delivery system: A review. American Journal of PharmTech Research 6(1): 2249-3387.
- Niehaus WG, S amuelson B (1986) Formation of MDA from phospholipids arachidonate during microsomal lipid peroxidation. European Journal of Biochemistry 6(1): 126-130.
- Osawa T, Kato Y (2005) Protective role of antioxidative food factors in oxidative stress caused by hyperglycemia. Annals of the New York Academy of Sciences 1043(8): 440-451.
- Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxides. Journal of Laboratory and Clinical Medicine. 70(1): 158-159.
- Racker E (1955) Glutathione reductase of N-acetyl-p-benzoquinone mine with reduced glutathione, acetaminophen and NADPH. Molecular Pharmacology 217(9): 855-866.
- Rahimi R, Nikfar S, Larijani B, Abdollahi M (2005) A review on the role of antioxidants in the management of diabetes and its complications. Biomedicine and Pharmacotherapy 59(7): 365 -373.
- Rang and Dale’s, pharmacology (7th)., pp. 377.
- Rojita M (2011) Antioxidants and their characterization. Journal of Pharmacy Research 4(8): 2744-2746.
- Salem Z, Yousry MN, Camacho LH (2013) Antioxidant and antibacterial activities of leaves and branches extracts of Tacoma stans juss. ex kunth against nine a species of pathogenic bacteria. African Journal of Microbiological Research 7(5): 418-426.
- Sang Hun Lee, Bong Hyun Jun (2019) Silver Nanoparticles: Synthesis and Application for Nanomedicine. International journal of molecular sciences 20(4): 865.
- Saqib Z (2005) Transdermal drug delivery of labetolol hydrochloride: System development, in vitro, ex vivo, in in vivo Current Drug Delivery 2(2): 125-131.
- Sarah W, Gojka Roglic, Anders Green, Richard Sicree, Hilary King, et al. (2004) Global Prevalence of Diabetes. Diabetes Care 27(5): 1045-1047.
- Siddiqi (2018) A review on biosynthesis of silver nanoparticles and their biocidal properties. Journal of Nanobiotechnology 6(1): 1-28.
- Sindhu M Tanu S (2013) Medicinal plants from twelve families having antidiabetic activity: A review. American journal of Pharmacy and Technology Research 3(5): 36-52.
- Singh PS, Raj Khanna, Jawahar L Kaw, Subhash K Khanna, Mukul Das, et al. (2003) Comparative effect of benzanthrone and 3bromobenznthrone on hepatic xenobiotic metabolism and anti-oxidative defense system in guinea pigs. Archives of Toxicology 77(2): 94-99.
- Sinha AK (1972) Colorimetric assay of catalase. Analytical Biochemistry 47(2): 389-394.
- Suresh B (2016) Diabetes: causes, symptoms and treatments. Public health environment and social issues in India. Chapter 5: 55-67.
- Szkdelski T (2001) The mechanism of alloxan and streptozotocin action in beta cells of the rat pancreas. Physiological Research 50(6): 536-546.
- Wild S, Gojka Roglic, Anders Green, Richard Sicree, Hilary King, et al. (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27(5): 1047-1053.

 Research Article
Research Article