ABSTRACT
Recently, the widespread use of probiotics (such as bifidobacteria) for health purposes has been reported. Currently, we are working on the development of a formulation that delivers live bifidobacteria to the intestines, with the aim of maximizing the use of probiotics. In this study, we examined the performance of this formulation through both nonclinical and clinical investigations. In the nonclinical investigation, we compared the disintegrability and bifidobacteria survivability of the acid-resistant and nonacid-resistant formulations under strongly acidic conditions. In the clinical trial, subjects were given an acid-resistant formulation containing live Bifidobacterium for 2 weeks, and fecal samples were taken before and after the intervention. Two methods were used to examine for the presence of live Bifidobacterium in the fecal samples. One is the culture method and the other is the method using PMA reagent. In each case, PCR was used to identify the species of Bifidobacterium. The disintegration time of the nonacid-resistant formulation was under 20 minutes in strongly acidic conditions (pH 1.2), whereas that of the acidresistant formulation was over 180 minutes under the same conditions. Furthermore, survive of Bifidobacterium in the acid-resistant formulation was confirmed even after the capsules were immersed in strong acid conditions for 120 minutes. In clinical study, Bifidobacterium, which were not detected in the pre-intervention fecal sample, were detected alive in the post-intervention fecal sample. The results suggest that this formulation protects the Bifidobacterium contained therein from acid and that Bifidobacterium may live and reach to intestines when taken orally.
Keywords: Bifidobacteria; Acid-Resistant Formulation; Survivability; Supplement; Clinical Study
Introduction
Over 100 trillion bacteria of more than 1,000 types live in the human intestine, forming the intestinal flora. Lactic acid bacteria and bifidobacteria-typically considered beneficial-lower the pH in the intestine by producing lactic acid and acetic acid, and suppress the growth of harmful bacteria and the production of putrefactive products. As a result, it has been reported to contribute to the prevention of illness and the maintenance of health [1,2]. Live bacteria that work beneficially to the host animal by improving the balance of intestinal bacteria are called “probiotics” and are used in various foods [3-5]. Bifidobacterium, a type of probiotic, is reported to exert various beneficial effects on the body through oral ingestion [6-9]; its main growth site is the large intestine. Orally ingested bifidobacteria are required to reach the intestines alive because it is important for live probiotics to act in the intestines. However, bifidobacteria are sensitive to acid, so when taken orally, they are affected by gastric acid. Therefore, there is concern that the viable cell count may decrease significantly when it reaches the lower part of the intestinal tract or growth site. Thus, when using bifidobacteria as probiotics, it is important to consider the acid resistance of the formulations. Although acid-resistant capsules are generally used to impart acid resistance to the formulation, it may be advantageous avoid using acid-resistant capsules when considering the leachability of other active ingredients. Therefore, we developed an acid-resistant formulation that contains both bifidobacteria and other active ingredients, without the use of acidresistant capsules. This study applied two methodologies in the form of nonclinical and clinical investigations to examine the acid resistance and bacterial survivability of this formulation.
Materials and Methods
Formulation
For the nonclinical investigation, we used two types of formulations; namely, a bifidobacteria-containing acid-resistant formulation (acid-resistant formulation) and bifidobacteriacontaining nonacid-resistant formulation (nonacid-resistant formulation). Hydroxypropyl methylcellulose (HPMC) and milk protein hydrolysates were used for the acid-resistant formulation, and crystalline cellulose was added instead for the nonacidresistant formulation. Bifidobacterium breve B-3(B-3; Morinaga Milk Industry Co., Ltd., Tokyo, Japan) and Bifidobacterium longum BB536 (BB536; Morinaga Milk Industry Co., Ltd.) live bacterial powder were used as probiotics; all other ingredients and quantities were the same. For the clinical study, a commercially available acidresistant formulation containing B-3 and BB536 (Naishi Support; FANCL Corporation, Kanagawa, Japan) was used as the test food. The number of viable bacteria contained in the daily test food was 5 billion for B-3 and 10 billion for BB536. The capsule used was a nonacid-resistant hard capsule (Vcaps PLUS; Capsugel, New Jersey, United States) containing HPMC as the main component.
Nonclinical Study
A disintegration test was conducted in accordance with the 17th edition of the Japanese Pharmacopeia [10]. A disintegration tester (NT-40HS; Toyama Sangyo Co., Ltd., Toyama, Japan) was used to measure the time taken for the formulations to disintegrate in solution 1 (pH 1.2) and solution 2 (pH 6.8) of the Japanese Pharmacopoeia at 37°C. The auxiliary board was not used. The judgment of disintegration was not when the outer capsule was dissolved, but when the mass of contents was dispersed in pieces; disintegration was observed up to 180 min. Capsules treated with solution 1 for 120 minutes were suspended in saline, mixed with TOS propionate agar medium (TOSP medium; Yakult Pharmaceutical Industry Co., Ltd., Tokyo, Japan), and cultured at 37°C for 72 hours under anaerobic conditions (AnaeroPack Kenki, Mitsubishi Gas Chemical Company, Inc., Tokyo, Japan). After culturing, the colony count was measured.
Clinical Study
Prior to its implementation, the clinical study was reviewed and approved by the Kobuna Orthopedic Clinic Ethics Committee (Tokyo, Japan, date of approval: March 4, 2021). The study was conducted in accordance with the Declaration of Helsinki (adopted in 1964, revised to its 7th Amendment at the WMA General Assembly of 2013 in Fortaleza) and the Ethical Guidelines for Medical and Health Research Involving Human Subjects (adopted December 22, 2014 and partially revised on February 28, 2017) to protect the human rights of subjects, and under the supervision of a physician. Participants were informed regarding the purpose and details of the study, providing voluntary, written consent for participation. This study was registered in the UMIN Clinical Trials Registry (UMIN 000043672).
Study Subjects
The subjects were healthy males and females aged 20–64 years who were capable of normal defecation on a daily basis. The exclusion criteria were follows: diarrhea or constipation; user of antibiotics; user of drugs that may affect digestion and absorption; being unable suspend the use of drugs, quasi-drugs, and health foods (foods containing B-3 and BB536) that may affect the study outcomes; food allergies; heavy drinking; diseases or serious complications requiring urgent treatment; gastrointestinal diseases or history of surgery that may affect digestion and absorption or defecation; irritable bowel syndrome; pregnancy or lactation; addiction to drugs and/or alcohol; participation or intent to participate in another clinical study; and being deemed ineligible for participation at the discretion of the principal investigator. The work associated with the management of subject recruitment was outsourced to the contract research organization KSO Co., Ltd. (Tokyo, Japan).
Study Method
The study was a single-group before-and-after study design; 10 subjects, selected at the discretion of the physician among 30 consenting candidates based on the background information obtained, were enrolled in the study. The intervention period was 2 weeks and subjects took 3 capsules daily after dinner. During the study (from provision of consent to the end of final tests), subjects were prohibited from consuming foods containing B-3 and BB536, as well as all health foods. For a total of 4 weeks-including the 2 weeks of pre-observation and 2 weeks of intake-subjects were asked to maintain their lifestyle habits. They were prohibited from consuming large amounts of alcohol (20 g/day of pure alcohol) and instructed to avoid using drugs—such as intestinal regulators and laxatives-that may affect bowel movements. They were also prohibited from drinking alcohol the day before and on the day of testing, as well as from smoking on the day of testing.
Survey and Test Items
As part of the background investigation, we collected data regarding sex, age, BMI, defecation status, health condition, use of drugs, use of supplements, details of diet, consumption of alcohol, and presence of food allergies. Additionally, subjects were asked to a keep daily record of whether or not they ingested the test food, changes in physical conditions, lifestyle changes, use of drugs and medicines, and the details of their diet and meals (including alcohol consumption) during the pre-observation and intervention periods. Subjects provided fecal samples both before and after the intervention period; these were used to determine the presence or absence of viable B-3 and BB536 in feces, as well as to measure the bacterial count. Subjects placed the feces in a sealable container and submit it to the testing facility (Kyoto Institute of Nutrition & Pathology, Inc., Kyoto, Japan) within 24 h after defecation, in cold storage and under anaerobic conditions.
Confirming the Presence or Absence of Live Bacteria
The presence or absence of live B-3 and BB536 in fecal samples was determined using a combination of the plate smear culture method and real-time quantitative polymerase chain reaction (RT-qPCR; culture-PCR method). After determining the total fecal weight, 1.0 g of homogenized feces was diluted stepwise in an anaerobic sample diluent (composition per 1L: 3.0 g Na2CO3, 0.9 g NaCl, 0.45 g K2HPO4, 0.45 g KH2PO4, 0.9 g (NH4)2SO4, 0.19 g MgSO4 ・7H2O, 0.12 g CaCl2・2H2O, 0.5 g L-Cysteine・HCl・H2O, 0.001 g resazurin, 0.5 g Agar, 50 μL Antifoam emulsion); 100 μL of the 10-5 diluted fecal solution was smeared onto TOSP medium and subjected to static culture for 48 hours at 37℃ under anaerobic conditions. After the culture period, the colony was recovered using a scraper and suspended in saline; 20 μL of the suspension was used for DNA extraction and RT-qPCR.
Bacterial Count Measurement
The total bacterial count (the total number of live and dead bacteria) of B-3 and BB536 in feces was measured according to the following procedures: 900 μL of phosphate buffered saline (PBS) was added to 100 mg of homogenized feces and thoroughly suspended until there were no more lumps (hereinafter, fecal suspension). To 200 μL of the fecal suspension, 800 μL of PBS was added before stirring and centrifuging the mixture (13,000×g, 5 min, 4°C); DNA was extracted from the remaining residue (equivalent to 20 mg of feces) after removing 800 μL of the supernatant, and was subsequently used for RT-qPCR. Next, we attempted to detect and quantify live bacteria in feces using propidium monoazide (PMA), an intercalating agent that binds to DNA (hereinafter, PMA-qPCR method). PMA, a selective membrane-permeable dye, invades the double-stranded DNA of dead cells with broken membrane integrity, binds to DNA by light irradiation, and inhibits the PCR reaction. Therefore, PMA treatment inhibits the PCR amplification of dead cells, enabling the specific quantification of viable cells [11]. To 200 μL of the previously obtained fecal suspension, 1 μL of PMAXX TM (20mM, BTI; Biotium, Inc., California, United States) was added. A suspension was produced by pipetting, stirred using a vortex as appropriate, and left to stand for 10 min at room temperature. The suspension was subsequently irradiated for 10 min with an LED Crosslinker12 (Takara Bio Inc., Shiga, Japan) and centrifuged (13,000×g, 5 min, 4°C), after which DNA was extracted from the remaining residue (equivalent to 20 mg of feces) after removing the supernatant; the extracted DNA was subsequently used for RT-qPCR.
DNA Extraction and RT-qPCR
DNA was extracted from samples using the QuickGene-810 system (Kurabo Industries Ltd., Osaka, Japan) and QuickGene DNA tissue kit (Kurabo Industries Ltd.) following previously reported procedures [12]. In particular, 250 μL of tissue lysis buffer (MDT) was added to a conical tube packed with beads containing the sample; the crushing process was performed twice at 3,000 rpm for 2 min using the Micro-Smash MS-100 (TOMY SEIKO Co., Ltd., Tokyo, Japan), producing a crushed solution. To this crushed solution, 25 μL of Proteinase K (EDT) was added, mixed, and left to stand for 2 h in a 55°C dry bath. The solution was then centrifuged (15,000×g, 10 min, room temperature), and 180 μL of lysis buffer (LDT) was added to 200 μL of the supernatant and mixed; the mixture was then left to stand for 10 min in a 70°C dry bath. After standing, 240 μL of 99.5% EtOH was added to the mixture, stirred well, and centrifuged (8,000×g, 1 min, room temperature), before purifying the supernatant using the QuickGene-810 system and its accompanying filter. After purification, the DNA extract was eluted with an elution buffer (CDT) and stored at -20℃ until use.
RT-qPCR was performed using the extracted DNA as a template, as well as B-3-specific primer (forward, GCGATACTGCCAGAACACCT; reverse, CACTATCAACGGCAGATTTCG) and BB536-specific primer (forward, GAACAGGGTGTGCTGAGTGA; reverse, CAAGCGAGAAGATCATCGAA) [13,14]. TB Green® Premix Ex Taq™ (Tli RNase H Plus; Takara Bio Inc.) was used to perform RT-qPCR. The reaction mixture comprised 5 μL of TB Green® Premix Ex Taq™ (Tli RNase H Plus 2×), 0.04 μL of each primer (50 μM), and 0.5 μL of the DNA extract, adjusted with sterile distilled water to a total volume of 10 μL. The PCR conditions were as follows: initial denaturation at 95°C for 30 seconds, followed by 30 repeated cycles of 95°C for 3 s and 60°C for 30 s. Samples wherein the amplification of PCR products was confirmed by 30 cycles were deemed positive, while samples wherein the amplification was not confirmed were deemed negative (below the detection limit). The bacterial count per 1 g of feces was calculated by creating a calibration curve using the DNA extracted from B-3 and BB536 raw material with known bacterial counts.
Results
Nonclinical Study
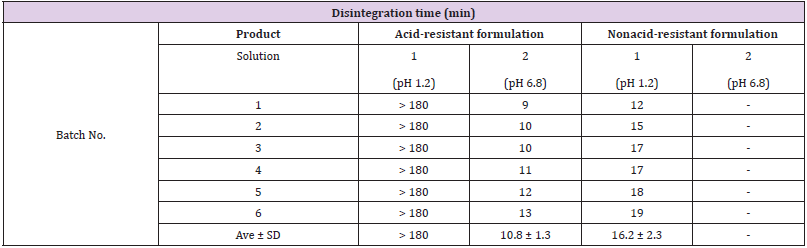
Table 1 shows the formulation disintegrability results, while Table 2 shows the bacterial survivability (live bacterial count) results in the nonclinical study. The disintegration time in solution 1 was 180 minutes or longer (did not disintegrate by 180 minutes) for the acid-resistant formulation, and 16.2±2.3 minutes for the nonacid-resistant formulation. When the test formulations were immersed in solution 1, the outer capsule gradually dissolved, but in the acid-resistant formulation, the powder in the capsule formed a lump and maintained the lump until for 180 min. After transfer to solution 2 following treatment with solution 1, the formulation disintegrated after 10.8±1.3 minutes. Since the nonacid-resistant formulation disintegrated completely in solution 1, it was not subjected to disintegration testing in solution 2. Results following treatment with solution 1 revealed that the live bacterial count of the acid-resistant formulation was 8.2×108 cfu/g, while that of the nonacid-resistant formulation was less than 1.0×105 cfu/g.
Table 2: Number of Bifidobacteria after treatment with the 1st fluid.
Note: cfu: Colony Forming Unit
Clinical Study
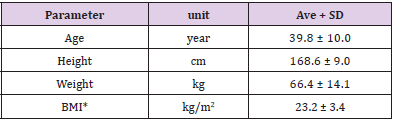
Table 3 shows the background characteristics of the subjects who participated in the clinical study (n=10). The average age was 39.8±10 years, and the average BMI was 23.2±3.4 kg/m2. All subjects completed the study, and since it was confirmed that there were no issues with the test food intake rate (more than 90%) and compliance, we included the data from all subjects in the analysis.
Culture Method
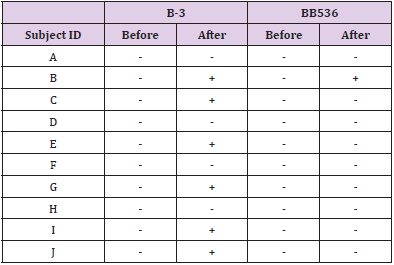
In a culture experiment using fecal samples collected from the subjects, colony growth was confirmed on all plates. As a result of RT-qPCR using colony-derived DNA, B-3 and BB536 were all negative before the intervention. On the other hand, after the intervention, B-3 was positive in 6 subjects and BB536 was positive in 1 subject (Table 4).
Table 4: Detection of live B-3 and BB536 bacteria of each subject in the fees (culture method).
Note: +: Detect
-: Not detect (Below the detection limit).
PMA Method
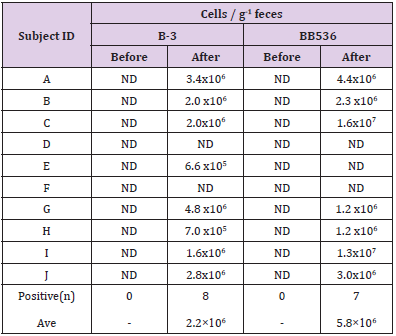
Table 5: Number of Bifidobacteria in faces (without PMA treatment).
Note: ND: Not Detect (<1.0×105).
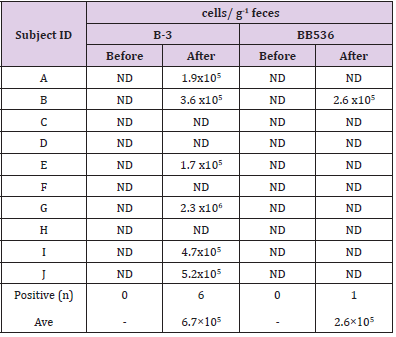
The results of RT-qPCR without PMA treatment are shown in Table 5. Both B-3 and BB536 were negative in feces before intervention. On the other hand, the postintervention fecal samples of 8 subjects tested positive for B-3, while those of 7 subjects tested positive for BB536. The average total cell count per 1 g of feces was 2.2×106 cells/g (n=8) for B-3 and 5.8×106 cells/g (n=7) for BB536. Table 6 shows the results of RT-qPCR performed on fecal samples that underwent PMA treatment. The preintervention fecal samples from all subjects tested negative for both B-3 and BB536. Conversely, the postintervention fecal samples of 6 subjects tested positive for B-3, while that of 1 subject tested positive for BB536. Additionally, the average cell count per 1 g of feces in positive subjects was 6.7×105 cells/g (n=6) for B-3 and 2.6×105 cells/g (n=1) for BB536.
Table 6: Number of Bifidobacteria in faces (without PMA treatment).
Note: ND: Not Detect (<1.0×105).
Discussion
In this study, we used an acid-resistant formulation containing live bifidobacteria to evaluate the acid resistance and bacterial survivability of the formulation through both a nonclinical and clinical approach. The results of the nonclinical investigation indicated that the acid-resistant formulation formed a powderous lump after dissolution of the coating capsule in a strongly acidic environment, protecting the bifidobacteria contained within the acid for more than 180 min. Since it has been reported that the residence time of regular food in the stomach upon ingestion is approximately 2 to 3 h [15], our results suggest that if the acidresistant formulation is ingested along with food, it is very likely to reach the intestinal tract, with the bifidobacteria on the inside protected from gastric acid. Conversely, the disintegration time of the nonacid-resistant formulation was around 16 min; thus, we hypothesized that it would disintegrate in the stomach, and the gastric acid would kill the bifidobacteria when the formulation is ingested along with food. Furthermore, as the powderous lump disintegrated around 11 min after being transferred to solution 2 following treatment with solution 1, we believe that the powderous lump that reaches the intestinal tract would gradually release bifidobacteria in the intestines. Additionally, from the results of the culture experiment using the formulation after treatment with solution 1, it was considered that the released bacteria contained many viable bacteria. The fact that live B-3 and BB536 bacteria were detected in fecal samples after subjects had ingested the test food in the clinical study supports the hypothesis obtained from the nonclinical study; furthermore, no live bacteria were detected in the preintervention fecal samples, supporting the notion that that the live bacteria detected post intervention came from the test food.
In the clinical study, aside culture method, we measured both the total number of B-3, BB536 and the number of live B-3, BB536 using the PMA reagent. Following RT-qPCR conducted using fecal samples not treated with PMA, the average bacterial counts (total number of dead and live bacteria) were 2.2×106 cells/g of feces for B-3 and 5.6×106 cells/g of feces for BB536. The ratio of B-3 and BB536 contained in the test food was 1:2, while the ratio of total B-3 and BB536 in feces was 1:2.5; therefore, we were able to observe a correlation between the ingested and excreted bacterial levels. On the other hand, in the fecal samples treated with PMA indicated that the average viable bacterial count was 6.7×105 cells/g of feces for B-3 and 2.6×105 cells/g of feces for BB536. As PMA treatment renders the DNA of dead bacteria unreactive to PCR, the viable bacterial count decreased to approximately 1/10 of the total bacterial count. Since the detection limit of the RT-qPCR method is approximately 1.0×105–106 copies per gram of feces [16], it is considered that many of the subjects remained below the detection limit, particularly regarding live bacterial detection.
The balance in the feces was higher for BB536 in terms of total bacteria count and for B-3 in terms of viable bacteria count. This suggests that there is a difference in the survival rate of BB536 and B-3 in feces during the period of transportation or storage after excretion. The conventional culture method, as a way to detect live bacteria in feces samples, has several disadvantages such as complexity of procedure, long culture time, presence of unculturable microorganisms and inability to use feces samples stored in freezing conditions, while the PCR method is burdened with the disadvantage of not being able to distinguish between dead and live bacteria. The approach to live bacteria detection using the PMA reagent that was adopted in this study, although not imparting absolute selectivity to live bacteria, produced a high concordance rate between the rate and number of positive results between the culture method and PMA-PCR, which means that one important outcome of this study is that it demonstrated the usefulness of PMA-PCR as a method of detecting live bifidobacteria in feces.
One of the research limitations of this study is that the conditions for the intake of the test foods were varied because the diet during the intervention period was not standardized. Furthermore, in consideration of the burden on the subjects, only one feces sample was taken after the intervention, so there is a possibility that feces with low viable bacteria count was submitted accidentally. Currently, there is no other way to confirm that orally ingested bacteria have reached the intestine alive than to detect viable bacteria in feces. However, there is a risk that this method may underestimate the rate of viable bacteria reaching the intestine, because the viability in the intestine does not always match the viability in the feces. In the future, in addition to the issues of clinical trials, more simple, accurate, and sensitive technologies are expected to be developed for viable bacteria detection methods. This study examined the properties of an acid-resistant formulation containing bifidobacteria and the viability of the bacteria in an acidic environment. The results indicated that the formulation formed a powderous lump under strongly acidic conditions, protecting the bifidobacteria from gastric acid for over 3 h. It was also confirmed that live bifidobacteria derived from the test food were detected in the feces of the subjects when orally ingested the formulation. These results suggest that this formulation is effective in protecting bifidobacteria from acid and delivering them alive to intestinal tract.
References
- Ljungh A, Wadström T (2006) Lactic acid bacteria as probiotics. Curr Issues Intest Microbiol 7(2): 73-89.
- Choi CH, Chang SK (2015) Alteration of Gut Microbiota and Efficacy of Probiotics in Functional Constipation. J Neurogastroenterol Motil 21(1): 4-7.
- Mitsuoka T (2002) Research in intestinal flora and functional foods. J Intest Microbiol 15: 57-89.
- R Fuller (1989) Probiotics in man and animals. J Appl Bacteriol 66(5): 365-378.
- Mitsuoka T (2000) Significance of dietary modulation of intestinal flora and intestinal environment. Biosci Microflora 19(1): 15-25.
- Benno Y, Mitsuoka T (1992) Impact of Bifidobacterium longum on human Fecal Microflora. Microbiol Immunol 36(7): 683-694.
- Minami J, Iwabuchi N, Tanaka M, Yamauchi K, Xiao J, et al. (2018) Effects of Bifidobacterium breve B-3 on body fat reductions in pre-obese adults: A randomized, double-blind, placebo-controlled trial. Biosci Microbiota Food Health 37(3): 67-75.
- Xiao J, Katsumata N, Bernier F, Ohno K, Yamauchi Y, et al. (2020) Probiotic Bifidobacterium breve in improving cognitive functions of older adults with suspected mild cognitive impairment: A randomized, double-blind, placebo-controlled trial. Journal of Alzheimer's Disease 77(1): 139-147.
- Ogata T, Kingaku M, Yaeshima T, Teraguchi S, Fukuwatari Y, et al. (1999) Effect of Bifidobacterium longum BB536 yogurt administration on the intestinal environment of healthy adults. Microb Ecol Health Dis 11(1): 41-46.
- (2016) Ministry of Health, Labour and Welfare. The Japanese Pharmacopoeia (17th)., pp. 140-141.
- Elizaquível P, Aznar R, Sánchez G (2013) Recent developments in the use of viability dyes and quantitative PCR in the food microbiology field. J Appl Microbiol 116(1): 1-13.
- Tsukahara T, Inoue R, Nakayama K, Inatomi T (2018) Inclusion of Bacillus amyloliquefaciens strain TOA5001 in the diet of broilers suppresses the symptoms of coccidiosis by modulating intestinal microbiota. Anim Sci J 89(4): 679-687.
- (2017) Morinaga Milk Industry Co., Ltd. Oligonucleotides. Patent Application No. 2017-216980(P2017-216980A). 2017-12-14.
- Rougé C, Goldenberg O, Ferraris L, Berger B, Rochat F, et al. (2010) Investigation of the intestinal microbiota in preterm infants using different methods. Anaerobe 16(4): 362-370.
- Hosoya N (supervision) (2002) Newly Revised Edition: Digestion and Absorption-Basic and Clinical-Daiichi Shuppan, p. 55.
- Matsuki T, Watanabe K, Fujimoto J, Takada T, Tanaka R (2004) Use of 16S rRNA Gene-Targeted Group-Specific Primers for Real-Time PCR Analysis of Predominant Bacteria in Human Feces. Appl Environ Microbiol 70(12): 7220-7228.

 Research Article
Research Article