Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Rasit Dinc*
Received: October 15, 2023; Published: November 01, 2023
*Corresponding author: Rasit Dinc, INVAMED Medical innovation Institute, Mutlukent Mah. 1961 Cd. No.27 06810 Cankaya, Ankara, Turkey
DOI: 10.26717/BJSTR.2023.53.008417
Coronary artery disease (CAD) remains as a cause of the leading mortality and morbidity worldwide despite advances in diagnosis and treatment. Currently, the stent applications are at the forefront of the strategies in the prevention of the stenosis in coronary arteries. However, restenosis, the re-narrowing of lumen diameter after percutaneous coronary intervention (PCI), is a significant problem. It commonly requires reintervention with PCI or coronary artery bypass grafting (CABG) for revascularization. Its underlying mechanisms are complex and not yet fully. Besides, the mechanisms between the native and the intervention-depended stenosis are significantly different. Three basic mechanisms that narrow the lumen due to PCI are elastic recoil (ER), vascular remodeling (especially for balloon angioplasty) and neointimal hyperplasia (mainly for stenting, including bare metal and drug-eluting stents). To overcome the stenosis, several PCI modalities have been developed using different platforms with different design, particularly drug-eluting stents (DES). Even though, the rates of post-stent stenosis are still not negligible. The stents have been used for decades as a life-saving strategy. Although, they still have significant research potential to find the one that meet the deliverability, efficacy, and safety characteristics. In this study, it was reviewed general pathophysiology and morphological features of post-restenosis in the technological advancements associated with the coronary intervention.
Keywords: Coronary Artery Diseases (CAD); Restenosis; In-Stent Atherosclerosis; Drug- Eluting Stents (DES); Pathophysiology
Abbreviations: CAD: Coronary Artery Disease; PCI: Percutaneous Coronary Intervention; POBA: Plain Old Balloon Angioplasty; ER: Elastic Recoil; ISR: In- Stent Restenosis; CABG: Coronary Artery Bypass Grafting; BMS: Bare Metal Stents; DES: Drug-Eluting Stents; ST: Stent Thrombosis; OCT: Optical Coherence Tomography; VSMC: Vascular Smooth Muscle Cell; SMC: Smooth Muscle Cells
Coronary artery disease (CAD) is a serious problem in which the coronary arteries cannot supply enough oxygen-rich blood to the heart due to plaque buildup in their walls. In the United State, CAD is most common type of heart disease and approximately 18.2 million American adults suffer from this disease [1]. Despite advances in diagnosis and treatment, it continues to be the leading cause of mortality and morbidity worldwide [2]. The first coronary angioplasty performed by Dr. Gruentzig in 1978 was a groundbreaking work in coronary revascularization after surgical bypass grafting, the only method previously [3]. Angioplasty, the first of the percutaneous coronary intervention (PCI) and later called plain old balloon angioplasty (POBA), was found effective in provide vascular patency of the narrowed intracoronary lumen size [4]. Unfortunately, the increased lumen diameter size after PCI was triggered by balloon injury in 32-55% of patients and narrowed again [5,6]. This phenomenon of lumen diameter reduction after PCI is well known and is defined as restenosis. This early restenosis formation is primarily due to vascular remodeling/elastic recoil (ER) in the pre-stent period [6]. Intense efforts are being made to prevent the stenosis problem and various strategies are being explored. Knowing the pathophysiology and mechanism of stenosis makes a significant contribution to the treatment of CAD [7]. In this regard, the stent applications are at the forefront of the strategies in the prevention of the stenosis problem [5,8,9]. In this review, an update on the pathophysiology and morphological features of in- stent restenosis (ISR) has been made to provide an overview for the reader as the concerns after stent intervention persist despite advances in the technological advancements associated with the coronary intervention.
ISR is a serious clinical problem and often requires reintervention with PCI or coronary artery bypass grafting (CABG) for revascularization. For this reason, most of the studies carried out in recent years have aimed to prevent its formation [10]. Bare metal stents (BMS) with permanent support structures, developed to overcome the stenosis phenome in POBA under the leadership of Sigwart et al. [11], are still widely used [12]. But the phenomenon of restenosis occurred in 17-41% of the BMS- implanted patients because of two major problems, stent thrombosis and ISR [6,8,13]. As a result of attempts to reduce restenosis, drug-eluting stents (DES) were started to be used as an alternative to BMS in the 2000s [14]. The special drugs (sirolimus, paclitaxel, etc.) on these stents are drugs that are also used in cancer treatment and prevent cell proliferation, including vascular endothelial cells to provide the reduction of ISR [15]. On the other hand, the prevent of vascular endothelial cell proliferation led to contact between the metal surface of the stent and blood for a longer period. Therefore, this first-generation DES (G1-DES) increased late ISR and late stent thrombosis (ST) that giving rise to significant influence on patient’s long-term clinical outcomes [9,16]. To overcome these unfavorable effects, the second-generation DES (G2-DES) have been developed using different platforms (cobalt-chrome etc.), with either more biocompatible durable polymers or bioabsorbable polymers 8 (Table 1).
Table 1: The timeline and brief view on important characteristic of the cardiovascular stents [8,18,19,37].
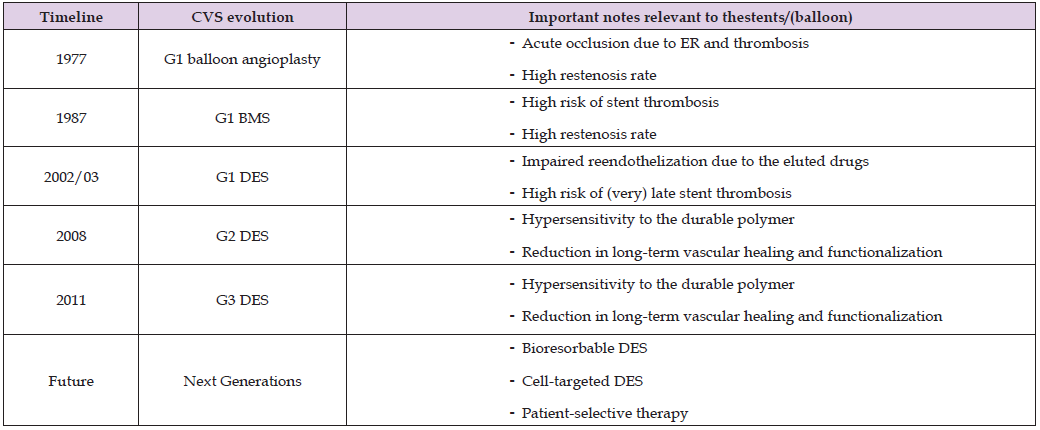
Note: *CVS: cardiovascular stent; G1, 2, 3: Generation 1st, 2nd, 3rd; BMS: Bare metal stents; DES: Drug-eluting stents; ER: elastic recoil.
Poly (lactic acid-co-glycolic acid) (PLGA), has been extensively used for the controlled drug-delivery, is a both biodegradable and biocompatible polymer [17]. Although DESs, especially the 2nd generation, reduce the rate of restenosis by <10%, the rate of ISR is still not negligible [2,6]. Future studies are designed to further reduce or even completely resolve complications such as hypersensitivity and material removal from the surface, especially due to the structural feature of the stent material. Aiming to prevent the hypersensitivity reaction, non-polymeric or biodegradable polymeric third-generation DES (G3-DES) with a semisynthetic analogue of sirolimus were also developed to replace with the durable polymeric ones [18]. Additionally, recently new generation DES composed of fully bioresorbable scaffolds BRS) has been constructed to provide vascular support and deliver the antiproliferative drug to inhibit neointimal proliferation for a given period. These new generation DES provide gradual resorption without leaving any residual foreign matter [9,12]. Several studies have also been made on cell-selective DES and personalized therapy [8,19].
Etiology
Vascular stenosis remains an important clinical problem that narrows vessel lumen 10. It can be occurred not only due to native conditions, but also due to percutaneous intervention, including the stenting [5,20]. In the etiology of ISR, patient-related factors (age, diabetes mellitus, genetics, etc.), lesion-related factors (type, length, location of the lesion, arterial size, etc.), procedural factors (type, length, expansion size, number of stents, etc.) play role [6]. The underlying mechanisms in stenosis are complex and not yet fully understood [21-23]. Besides, the mechanisms between the native and the intervention-depended stenosis are significantly different [2,6]. Three basic mechanisms that narrow the lumen due to PCI are elastic recoil (ER), vascular remodeling and neointimal hyperplasia. ER is the difference in arterial lumen size caused by arterial elastin fibers in the internal and external elastic laminae following complete inflation or deflating of the balloon in the angioplasty intervention. Vascular remodeling is a natural response to injury to repair the injured artery 2. This review will not discuss these two mechanisms which related with POBA before the stent era. Whereas about the process of native atherosclerosis will be mentioned as a general summary in order to make the subject more understandable.
Native Atherosclerosis
Coronary native atherosclerosis that will eventually lead to stenosis is a physiological process that begins with intimal hyperplasia, which is formed by the natural accumulation of smooth muscle cells (SMC) in branches prone to atherosclerosis in early childhood [24]. However, intimal hyperplasia may progress to pathological thickening with the formation of extracellular matrix in addition to SMCs in advanced ages. Later, invasion of lipid pools by macrophage-derived foam cells into the intima forms early and late fibroatheromas accompanying large necrotic cores with the fibrous cap. The fibrous cap is a vital structure that protect the necrotic core. A thinning of the fibrous cap by macrophage proteases can cause plaque rupture resulting in induction de- endothelialization and platelet thrombosis [2,25].
Stent Dependent Occlusion
In-Stent Atherosclerosis: In-stent atherosclerosis or “neo-atherosclerosis” that will eventually lead to restenosis of the artery begins with the histological finding of lipid-laden foamy macrophages. This may sometimes be accompanied by a necrotic core and/or calcification within the neointima. The necrotic core is thought to confer from apoptosis of the macrophage-derived foam cells around the strut area or near the luminal surface. This necrotic core may progress to fibro atheromatous plaques over time. In addition, intraplaque hemorrhage may occur due to leakage from the lumen or adventitial vasa vasorum next to the struts, with or without fibrin deposits. Like in coronary native atherosclerosis, foamy macrophage infiltration from the neointima can cause thinning of the fibrous cap, leading to the plaque rupture and thrombosis [2,26,27]. Another feature of instent atherosclerosis is calcification, particularly associated with the long-term consequences of stent implantation. In-stent neointimal calcification is observed in patients with both BMS and DES implants. The calcification can differ morphologically from microcalcification to calcified sheet [2,26,28]. While coronary native atherosclerosis takes several years, in-stent atherosclerotic usually occurs in a shorter time after the PCI. Furthermore, the onset of in-stent atherosclerosis development is significantly earlier after the DES stenting (70 days for some DES) than after BMS stenting (900 days). Furthermore, the overall incidence of atherosclerosis is higher for DES than for BMS. Whereas this rate is approximately similar among G1-DES and G2- DES [2,26].
Neointimal Hyperplasia: The responsible mechanisms of neointimal hyperplasia are not fully understood. One of the major potential mechanisms is that stent implantation, including BMS, induces vascular injury that causes vascular smooth muscle cell (VSMC) proliferation and migration to the endothelial space 25. Again, circulating mitogens such as angiotensin II and plasmin may be involved in VSMC proliferation and migration due to overexposure based on endothelial denudation. DES with different properties has been developed to compensate for excessive neointimal proliferation. However, the problem could completely not be eliminated due to the increased rate of late restenosis [6]. The incomplete endothelial barrier also initiates the migration of lipoproteins within the lower endothelial space, initiating the atherosclerosis process [27,28]. In addition to these, the stent implantation also induces the migration of immune cells by activating the expression of cell adhesion molecules (such as ICAM-1, PECAM- 1 and VCAM-1) around the stent strut. Subsequently, monocytes adhering to these cell adhesion molecules migrate to the subendothelial space and transform into the foam cells. Moreover, polymers on DES can induce chronic inflammation characterized by infiltration of lymphocytes, macrophages, and giant cells that contribute to the vascular stenosis [29,30]. In addition, thrombosis associated with plaque rupture due to in-stent atherosclerosis. Even in rare cases, erosion can be seen regardless of the presence of intra- stent restenosis [31]. Briefly, the mechanisms underlying restenosis are considered to include the following steps: denutation of endothelial cells due to PCI, initiation of migration of inflammatory and/or lipoproteins/vascular smooth muscle cells, followed by neointimal hyperplasia and/or neoatherosclerosis (Figure 1) [32].
Intravascular ultrasound (IVUS), especially with virtual histology- IVUS, imaging provides real-time information about the composition, size and distribution of atherosclerotic plaque, as well as the luminal area. However, IVUS has some limitations in distinguishing neo-intimal tissues due to signal interference from the stent metal struts [6,33,34]. Restenosis requires revascularization by PCI or CABG. The recurrence of angina symptoms is clinically defined as restenosis that require revascularization 2. The imaging methods help us to classify restenosis: patent (<50% stenosis), intermediate stenosis (50- 75%), residual lumen (>75%), total occlusion 16. Re-narrowing of the lumen to ≥50% occlusion after PCI is evaluated as indication for revascularization [35]. With its significantly higher resolution capacity, optical coherence tomography (OCT) has become the outstanding method for the evaluation of restenotic tissue morphological features such as structure, backscattering, microvessels. In fact, the feature of being the outstanding method is not limited to these. OCT has also become the method of choice for the measurements of the peristrut neoatherosclerotic composition, including macrophage infiltration, lipid deposition, calcification, fibrous cap thickness, neointimal plaque rupture [36]. In the prevention and treatment of ISR, it has been started to rate different strategies such as pharmacological (lipid-lowering or antiplatelet therapy, agents targeting inflammation and oxidative pathways) and device-based (drug-eluting balloons, dedulking technologies and bioresorbable vascular scaffolds) [2].
An ideal stent should meet the three main categories involved of deliverability, efficacy, and safety. To detail further, an ideal stent should have good biocompatibility, flexibility, and strong radial force, and good radiopacity characteristics. In addition, it should not cause high rates of thrombogenesis, neointimal hyperplasia, inflammatory reaction, and stent thrombosis in the long term [37]. With new stent platforms which either using new materials or creating new designs, several novel strategies may be able to provide these features to a great extent. These novel strategies not only have new stent platform to functionalize stent surfaces, but also use more precise manufacturing technologies [37,38]. The advances in the manufacturing technologies seems to open the door for patient-specific devices, cell- selective DES, and bioresorbable DES [39,40]. Consequently, they will ensure to overcome the challenges of restenosis by preventing the mechanisms-above mentioned.
PCI play a vital role in CAD treatment. Nonetheless the BMS is still widely used, DES technology has begun to replace this stent. However, it has not been able to provide a permanent solution for restenosis and thrombosis. Future studies are designed to further reduce or even completely resolve complications arising from the structural characteristics of the stent material. Although stents have been used for decades, they still have significant research potential. Even though, it is predicted that the problem of in-stent restenosis encountered in the existing stents used today will be solved permanently. However, in order to achieve these goals, stent optimization must be succeeded at all relevant stages, from design, material selection and manufacturing method to the surface functionalization and implantation procedure.
The author declare that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The author thanks to Ahmet Umit Ardic for drawing the figure.
The authors received no extramural funding for the study.
RD drafted, conceptualized, and finalized the manuscript structure and contents.


