Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Isatta Wurie*, Foday Bangura, Osman Kargbo and Mohamed Samai
Received: July 27, 2023; Published: December 06, 2023
*Corresponding author: Isatta Wurie, College of Medicine and Allied Health Sciences, University of Sierra Leone, Sierra Leone
DOI: 10.26717/BJSTR.2023.54.008484
Introduction: This study aimed to determine the prevalence of hemoglobin variants, specifically sickle cell trait (SCT), among blood donors at Princess Christian Maternity Hospital (PCMH) and Ola During Children’s Hospital (ODCH) Blood Bank in Freetown. The screening included assessment of, the sensitivity and specificity of different testing methods, including wet preparations with sodium metabisulphite and electrophoresis, were compared.
Methodology: A total of 200 blood samples were collected from healthy blood donors who met the National Safe Blood Services’ criteria and had been screened for transfusion transmissible infections (TTIs). The samples were analyzed for sickle cells using wet preparations with sodium metabisulfite and microscopic assessment screening, as well as cellulose acetate electrophoretic separation. Blood group serotyping was also conducted for all samples and correlated with the donor chrateristics.
Results: Out of 200 donor, 183(92%) of them are Male participants, the remaining 17(9%) of the participants are Female. 96(48%) are in the age ranges are 18-25yrs old. Majority of the donor and the remaining 73, 18, 10, and 3(37%, 9%, 5%, and 2%) of the participant’s age groups are 26-35yrs, 36-45yrs, 46-55yrs, and 56- 69yrs old, 102(51%) belong to the blood group O+, and the remaining 47, 41, 6, and 4(24%, 21%, 3%, and 2%) of the donors blood groups are A+, B+, AB+, and O-. Sickle cell microscopic result showed. 157(79%) of the donors test are Negative, and the remaining 43(22%) Positive. The Haemoglobin (Hb) Electrophoresis using cellulose acetate paper showed. 154(77%) of the participants belong to the Cellulose Acetate Paper HB Electrophoresis group AA, and 45(23%) showed heterozygous AS and 1 (1%) AC. The minimum age group is 56-69yrs, and the maximum age group set is 56-69yrs (2%), with average age groups of 1.75 and a standard deviation of the age group set is 1.472.
Conclusion: This study revealed a 23% prevalence of sickle cell trait (SCT) among blood donors at the PCMH/ ODCH blood which is national blood transfusion center. The dynamics of the sickling component may not make it suitable for transfusion due to potential clogging of filter and also higher risk of the cells sickling and causing harm in intra-uterine or neonatal transfusion. Furthermore as with these factors emphasizes the need for screening blood donors for sickle cell to not only to avoid potential harm to both donors and recipients, but will also improve understanding of sickle cell trait donation and transfusion whilst ensure national sickle cell prevention programs are able to use in their prevention by raising awareness interventions.
Abbreviations: SCT: Sickle Cell Trait; PCMH: Princess Christian Maternity Hospital; ODCH: Ola During Children’s Hospital; SCD: Sickle Cell Disease; RBCs: Red Blood Cells; DHTR: Delayed Haemolytic Transfusion Reactions; PPE: Personal Protective Equipment; SOPs: Standard Operating Procedures
Introduction
Background: Haemoglobin (Hb) is a protein found in red blood cells (RBCs) responsible for carrying oxygen from the lungs to the tissues and organs of the body. Haemoglobinopathies are genetic disorders that affect the structure or synthesis of haemoglobin. The most common haemoglobinopathies worldwide are sickle cell disease (SCD) and thalassaemia. Sickle cell disease is an inherited disorder of the haemoglobin molecule that affects millions of people worldwide, particularly those of African descent (Piel [1]) and with a high prevalence in sub-Saharan Africa, the Middle East, and the Indian subcontinent (Weatherall [2]). The disease is caused by a mutation in the beta-globin gene, which leads to the production of abnormal hemoglobin (Hb) molecules that tend to form rigid, sickle-shaped red blood cells. These sickle cells can cause a range of health problems, including anemia, pain, organ damage, and increased susceptibility to infections (Rees [3]). SCD is a major public health concern in Sierra Leone, where it is estimated that up to 15% of the population may carry the sickle cell trait (Tshilolo [4]). In Sierra Leone, the prevalence of sickle cell disease is estimated to be 12-15% (Abdul-Karim et al.), making it one of the highest in the world. The disease causes chronic haemolytic anaemia, acute and chronic pain, and organ damage due to vaso-occlusion. The management of SCD requires a multidisciplinary approach, including transfusion therapy, pain management, and prevention and treatment of complications (Rees [3]).
Blood transfusion is a critical intervention for patients with sickle cell disease (SCD) as it can replace damaged red blood cells and prevent complications such as stroke or acute chest syndrome (Howard et al.) [5]. However, the success of transfusion therapy largely depends on the availability of compatible blood donors with normal hemoglobin (HbA) levels, which can be challenging in regions where SCD is highly prevalent (WHO [6]). It is estimated that 50-90% of patients with SCD will require at least one transfusion in their lifetime (Koshy, et al. [7]). Nonetheless, transfusion therapy can be complicated by the presence of haemoglobin variants in blood donors, which can affect the quality of transfused blood and cause adverse reactions in recipients (Osei-Kwasi). Transfusing sickle cell positive blood to a non-sickle cell patient can lead to various complications, including acute chest syndrome and delayed haemolytic transfusion reaction. These complications occur due to the abnormal hemoglobin present in sickle cell disease, which can cause blockages in blood vessels and lead to decreased oxygen supply to tissues. Therefore, it is essential to carefully consider the risks and benefits of transfusing sickle cell positive blood to a non-sickle cell patient and to explore alternative transfusion options when possible (NIH, 2021).
Justification: Due to the limited information available regarding the prevalence of sickle cell in Sierra Leone. The lack of knowledge regarding the specific phenotype of sickle cell makes it necessary to conduct research to quantify the number of healthy blood donors who may have the condition and are willing to donate blood to save lives. Currently, there is a lack of counseling services to educate individuals about sickle cell, leading to a rise in the disease's prevalence. The absence of routine sickle cell tests for blood donors puts them at risk of exposure to health complications. Integrating sickle cell tests into standard blood donor tests such as wet prep and Hb electrophoresis would aid in managing the condition and prevent further complications. The findings from this research can be utilized by the government to increase awareness about the rate of sickle cell trait and disease among healthy blood donors in the PCMH/ODCH blood transfusion centers, and to improve prevention, treatment, and management strategies for sickle cell in Sierra Leone.
Research Questions: The following research questions will guide this study:
1. What is the rate of haemoglobin variants (sickle cell) among blood donors at Princess Christian Maternity Hospital (PCMH) and Ola During Children’s Hospital (ODCH) Blood Bank Freetown?
2. What is the distribution of haemoglobin variants (sickle cell) among blood donors at Princess Christian Maternity Hospital (PCMH) and Ola During Children’s Hospital
3. What are the sensitivity and specificity of wet prep and electrophoresis methods?
4. What is the distribution of haemoglobin variants and ABO among blood donors at the PCMH and ODCH Blood Bank?
Problem Statement: The CENTRAL BLOOD BANK located at the Princess Christian Maternity Hospital (PCMH) and Ola During Children’s Hospital (ODCH) Blood Bank in Freetown is the major blood collection centers in Sierra Leone. This blood bank relies on both family replacement blood donors which make up of the largest percent (85-90%) of blood donor pool and voluntary blood donors to provide safe and compatible blood for patients in need. However, there is limited data on the prevalence of HbS among blood donors in this facility. Without this information, it is challenging to determine the risk of transfusing HbS-positive blood and to develop effective screening strategies.
Research Aim and Objectives: The aim of this study is to determine the rate of HbS among blood donors at PCMH and ODCH Blood Bank in Freetown. The specific objectives are:
1. To determine the prevalence of HbS among blood donors at PCMH and ODCH Blood Bank in Freetown.
2. To compare the rates of HbS between male and female blood donors.
3. To assess the age distribution of HbS-positive blood donors.
4. To determine the sensitivity and specificity of Wet Prep. Sodium Meta-bisulfite and Hb Electrophoresis methods.
Significance of the Study: This study will provide valuable data on the prevalence of HbS among blood donors in Freetown, Sierra Leone. The findings will inform the development of screening strategies to ensure safe blood transfusions and reduce the burden of SCD in the country. Additionally, the study will contribute to the body of knowledge on the epidemiology of HbS among blood donors at the PCMH and Ola During Blood Bank in Freetown, Sierra Leone.
Scope and Limitations of the Study: This study will focus on blood donors at Princess Christian Maternity Hospital and Ola During Children’s Hospital blood banks in Freetown, Sierra Leone. The study will use a cross-sectional design to collect data on the prevalence of Hb variants, including sickle cell, among blood donors, as well as their demographic and clinical characteristics and knowledge and attitudes towards sickle cell disease and blood donation. The study is limited by the sample size and may not be representative of the entire population of blood donors in Freetown, Sierra Leone.
Literature Review
Background: Haemoglobin variants are genetic disorders that affect the structure or production of haemoglobin, leading to abnormalities in the oxygen-carrying capacity of red blood cells. Sickle cell disease (SCD) is one of the most common haemoglobin variants worldwide, affecting millions of people, particularly those of African descent (Piel [1]). The sickle cell gene is carried by approximately 4% of the global population, with a prevalence of 25% in parts of sub-Saharan Africa (Weatherall [2]). Sierra Leone is one of the countries in West Africa with a high prevalence of SCD, and studies have shown that up to 15% of the population carries the sickle cell gene (Kallon et al.). Sickle Cell Disease (SCD) is an autosomal recessive disorder caused by a single nucleotide substitution in the beta-globin gene, resulting in the production of abnormal haemoglobin (HbS) (Steinberg [1]). HbS has a tendency to polymerize under low oxygen conditions, causing red blood cells to assume a sickle shape, become stiff and sticky, and block small blood vessels (Ware). This process leads to a wide range of clinical manifestations, including anaemia, vaso-occlusive crises, organ damage, and increased susceptibility to infections (Rees [3]). The prevalence of SCD and SCT varies widely across different regions of the world, depending on the frequency of the HbS gene in the population. In sub-Saharan Africa, for example, the prevalence of SCD is estimated to be 2-3% of live births, while the prevalence of SCT is much higher, ranging from 10-30% (Piel, et al.).
In the United States, the prevalence of SCD is lower, at approximately 1 in 500 African Americans, while the prevalence of SCT is higher, at approximately 1 in 12 African Americans (Hsieh, et al.). Blood transfusion is a crucial aspect of medical care for patients with SCD, as they often require frequent transfusions to manage their anemia and prevent complications such as stroke and organ damage. However, transfusions can also lead to complications, such as alloimmunization and iron overload, which can further exacerbate the morbidity and mortality associated with SCD (Vichinsky, et al.). Given the high prevalence of SCD and SCT in certain populations, it is important to understand the frequency of these hemoglobin variants among blood donors, as well as the potential risks and benefits of transfusing blood from donors with SCT or SCD. The current study aims to investigate the rate of HbS among blood donors at Princess Christian Maternity Hospital (PCMH) and Ola During Children’s Hospital (ODCH) Blood Bank in Freetown, Sierra Leone [8].
Epidemiology of Sickle Cell Disease: SCD is a major public health problem in many parts of the world, particularly in sub-Saharan Africa, where it is estimated that up to 2% of infants are born with the disease (Grosse, et al.). In Sierra Leone, the prevalence of SCD is estimated to be around 15-20% (Murray, et al.), making it one of the highest in the world. The high prevalence of SCD in Sierra Leone is thought to be due to a combination of factors, including a high rate of consanguineous marriages, limited access to prenatal diagnosis and genetic counseling, and a lack of comprehensive SCD prevention and management programs (Murray, et al.).
Prevalence of SCD and SCT Among Blood Donors: Several studies have investigated the prevalence of SCD and SCT among blood donors in different regions of the world. In a study conducted in Ghana, the prevalence of SCT among blood donors was found to be 25.9%, while the prevalence of SCD was 2.3% (Allain, et al.). Another study conducted in Nigeria reported a prevalence of 25.5% for SCT and 2.2% for SCD among blood donors (Ogunrinde, et al.). In the United States, the prevalence of SCT among blood donors is much higher, at approximately 8% among African Americans and 0.5% among Caucasians (Hsieh, et al.). The prevalence of SCD among blood donors is much lower, at less than 1% (Hsieh, et al.).
Haemoglobin Variants and Blood Donors: Haemoglobin variants are common in many populations worldwide, and the prevalence of different variants varies between populations. In sub-Saharan Africa, the prevalence of the sickle cell trait ranges from 10% to 40%, with the highest rates observed in West Africa (Grosse, et al.). The sickle cell trait is particularly common in Sierra Leone, where it has been estimated to affect up to 25% of the population (Koroma, et al.). Blood donors are an important source of blood for transfusion, and the prevalence of haemoglobin variants among blood donors can have significant implications for blood transfusion services. In particular, the presence of the sickle cell trait among blood donors can increase the risk of transfusion-associated sickle cell complications in patients with SCD (National Heart, Lung, and Blood Institute, 2012). Previous studies have investigated the prevalence of haemoglobin variants among blood donors in different populations. In a study conducted in Nigeria, the prevalence of the sickle cell trait among blood donors was found to be 23.2%, with a higher prevalence observed among female donors (Omotade, et al.). In another study conducted in Ghana, the prevalence of the sickle cell trait among blood donors was found to be 18.6% (Ansong, et al.).
Blood Donation and SCD: Blood transfusion is a life-saving intervention that requires a sufficient supply of safe blood. In Sierra Leone, blood transfusion is frequently required, with the most common indications being obstetric emergencies, malaria, and trauma (Levy, et al.). However, the prevalence of SCD in the population raises concerns about the safety of blood transfusion, particularly in areas where the availability of safe blood is limited. Studies have shown that the prevalence of SCD among blood donors in sub-Saharan Africa ranges from 3% to 35%, with higher rates reported in areas with a high prevalence of the sickle cell gene (Koura, Osei-Yeboah, et al.). Several studies have investigated the prevalence of HbS among blood donors in Sierra Leone, with varying results depending on the population and location of the study. For instance, a study by Kallon, et al. reported a prevalence of 7.8% among blood donors in a rural district hospital in Sierra Leone, while another study by Musa, et al. found a prevalence of 22.6% among blood donors in a tertiary hospital in Freetown. A more recent study by Fofanah, et al. reported a prevalence of 18.9% among blood donors at the PCMH blood bank, which is consistent with previous estimates [9].
Sickle Cell Anaemia Among Blood Donors: Blood transfusion is a life-saving intervention for patients with a range of medical conditions. However, the safety of transfused blood depends on the absence of infectious agents and the compatibility of the blood group and haemoglobin type. Carriers of haemoglobin variants, including SCA, can donate blood without exhibiting any symptoms, but the presence of abnormal haemoglobin in the donated blood can pose a risk to the recipient. The prevalence of SCA among blood donors varies widely depending on the geographic region and the screening methods used. Studies from sub-Saharan Africa have reported SCA prevalence rates ranging from 0.8% to 20% among blood donors (Makani, Kasvosve, Al-Sawafi, et al.). In the United States, where universal screening for SCA is recommended for all blood donors, the prevalence is estimated to be less than 1% (Hassell).
Screening for Haemoglobin Variants: Screening for haemoglobin variants is essential to ensure the safety of blood transfusion and to prevent the transmission of genetic disorders. Several screening methods are available, including electrophoresis, high-performance liquid chromatography, and molecular techniques (Weatherall). The most commonly used methods in Sierra Leone are Wet Prep and electrophoresis, which are simple and affordable technique that can detect the most common haemoglobin variants, including HbS.
Blood Donor Recruitment and Retention: Blood donor recruitment and retention are critical factors in ensuring a sufficient supply of safe blood. However, recruiting and retaining donors in low-resource settings can be challenging due to various factors, including low awareness, misconceptions, fear of adverse reactions, and inadequate incentives (Watt, et al.). Strategies to improve donor recruitment and retention include community-based mobilization, education and awareness campaigns, provision of incentives and recognition, and regular communication and follow-up (Newman, et al.).
Sickle Cell Disease And Blood Transfusion In Sierra Leone: In Sierra Leone, SCD is a major health burden, and blood transfusions are often necessary for the management of complications such as severe anemia, stroke, and acute chest syndrome (Huffman, et al.). However, the high prevalence of HbS in the population, coupled with a lack of resources and infrastructure for blood screening and typing, creates challenges for blood banks in ensuring the safety of blood transfusions (Huffman, et al.). A study conducted in 2016 by Tit man et al. found that only 7% of blood units in Sierra Leone were tested for haemoglobin variants, indicating a significant gap in blood safety practices in the country [10].
Blood Transfusion and Sickle Cell Variants: Blood transfusion is an essential component of medical care, particularly in the treatment of anaemia, trauma, and surgical procedures. However, transfusion of blood that contains sickle cell variants can lead to complications, such as Sickling of the red blood cells, acute chest syndrome, and delayed haemolytic transfusion reactions (DHTR). Therefore, it is important to screen blood donors for sickle cell variants to prevent these complications (Tchuenche, et al.).
Methodology
At the University of Sierra Leone, Teaching Hospital Complex - Princess Christian Maternity Hospital (USLTHC-PCMH)/ODCH Blood Bank, which is situated on Fourah Bay Road in Eastern Freetown, Sierra Leone, two hundred blood samples were collected from blood donors who had undergone all blood donation selection procedures, such as registration, pre-donation counseling, and screening. To determine the sickle cell status of each blood sample, a sodium metabisulfite screening test is conducted using a slide, coverslip, and microscope with a 40x lens. The results of the test can indicate whether the sample is positive or negative for sickle cell.
WET PREP (Sodium Meta-Bisulfite Method)
The wet prep (sodium meta-bisulfite method) is a screening test used to detect the presence of sickle-shaped red blood cells, which are characteristic of sickle cell disease. This method is based on the principle that sodium meta-bisulfite reduces the oxygen tension, inducing the sickle/crescent shape of red blood cells. The following procedure outlines the steps for performing the wet prep test.
Procedure:
1. Measure 0.2g of sodium meta-bisulfite using a weighing balance.
2. Add the measured sodium meta-bisulfite to 10ml of distilled water in a dry, clean, and sterile container, creating a 2% sodium meta-bisulfite working solution.
3. Mix the solution thoroughly and close the container.
4. Take one drop of blood sample and one drop of the working solution using a graduated dropper or pipette and place them on a microscopic slide.
5. Mix the blood sample and the working solution well for even distribution using a cover slip.
6. Cover the slide with the coverslip.
7. Wait for 15 minutes to allow the reaction to occur.
8. Examine the slide under a microscope using a 40x objective to observe the presence of crescent/sickle-shaped red blood cells.
9. Repeat the observation for one hour to confirm the findings.
Interpretation:
• If 20% or more of the red blood cells in each field show sickle-shaped morphology, the result is reported as positive.
• If fewer or no sickle-shaped red blood cells are observed, the result is considered negative.
Quality Control:
• Perform sickling controls (positive samples) each day before analyzing the samples to ensure the validity of the test.
• Solubility testing can be conducted on the positive control samples to further confirm the presence of sickle cell disease.
Precautions:
• Prepare a fresh working solution of sodium meta-bisulfite every day to maintain the accuracy of the test.
• Ensure the working solution is thoroughly mixed before use.
• Clean any excess blood from the coverslip using cotton wool before covering the slide.
• Remove any excess blood on the slide by applying cotton wool to each side.
• Properly label all working reagents and slides before use.
• Clean the microscope and working area before and after performing the test.
Cellulose Acetate Paper
Hemoglobin Electrophoresis: Hemoglobin Electrophoresis is an important diagnostic test and is considered the gold standard for confirming sickle cell anemia.
a) Principle:
The principle of this test lies in the fact that hemoglobin, a protein found in red blood cells, carries a negative charge at alkaline pH. When subjected to electrophoresis, which involves the application of an electric field, hemoglobin molecules will migrate toward the anode, which is the positive end of the electrophoresis setup. This characteristic movement of hemoglobin proteins allows for their separation and identification, aiding in the diagnosis of various hemoglobinopathies, including sickle cell anemia [11].
Procedure:
1. Prepare the buffer mixture:
• Mix 50 ml of Buffer 1 and 50 ml of Buffer 2 in a small dish.
2. Soak the cellulose acetate strip:
• Float the cellulose acetate strip in the buffer mixture, ensuring it is completely wet.
• Allow the strip to soak for 2-3 minutes.
3. Remove excess buffer:
• Take out the strip from the buffer mixture.
• Blot the excess buffer from the strip using filter papers.
4. Prepare the blood sample:
• In a test tube, mix 3 drops of distilled water and 2 drops of whole blood thoroughly.
• Allow the mixture to sit for approximately 5 minutes.
5. Apply the blood sample
• Using capillary tubing, apply a thin line of the lysed blood across the middle of the strip.
• The strip allows for up to 8 samples, and a ruler can be used to ensure straight bands.
6. Set up the electrophoresis tank:
• Carefully open the cover of the electrophoresis tank.
• Invert the strip and place it horizontally inside the tank.
• Ensure the strip is well covered by the buffer solution.
7. Start the electrophoresis process:
• Turn on the power pack.
• Set the current to 9-14 mA at 200 volts.
• Set the timer for 1 hour and 20 minutes, then press start.
8. Monitor the separation of hemoglobin bands:
• After approximately 30 minutes, the hemoglobin bands will start to separate.
• Faster hemoglobin variants will move toward the anode, while slower ones will remain closer to the cathode.
• Once the designated time has elapsed, turn off the electric current.
9. Remove and examine the strip:
• Take out the strip from the tank.
• Invert it again, turning to the opposite end for better examination.
• Observe and compare the bands on the strip to the controls.
Interpretation of Results:
• HbAA: bands that remained at the cathode side.
• HbAS: double bands, one at the cathode end and another at the anode side
• HbSS: Bands that migrate toward the anode.
Quality Control: To ensure quality control during the testing process, abnormal hemoglobin samples, specifically HbAS and HbSS, were concurrently applied with negative samples (AA) alongside the test samples for the respective day.
Precautions:
1. Proper Labeling: Each strip was carefully labeled and an arrow indicating the direction of sample placement was clearly marked. This ensured the accurate identification and organization of the samples for analysis.
2. Thorough Washing: After the capillary tubing was used to collect each sample, it was diligently washed with distilled water. This step was crucial to remove any residue or contaminants that could interfere with the accuracy of the subsequent analysis.
3. Controlled Moisture Level: Special attention was given to the cellulose paper used for the experiment. It was ensured that the paper was not completely dry before applying the sample bands. This precaution prevented any undesired effects on the migration of the samples during the analysis.
4. Accurate Record-Keeping: Precise records were maintained throughout the experiment in accordance with the designated strip placements. This meticulous approach ensured the integrity of the data collected and enabled a reliable analysis of the results [12-15].
Study Design
The objective of the research is to obtain 200 blood samples from individuals who have donated blood at the Princess Christian Maternity Hospital (PCMH) Blood Bank. These samples will be collected from both family replacement donors and voluntary blood donors who are between the ages of 18 to 60 years and have given their consent to participate in the study through the Blood Service donor drive protocol. The study aims to investigate the prevalence of the hemoglobin S variant among blood donors using Fischer's formula as a convenient sampling method. Only eligible subjects meeting the inclusion criteria will be included in the study, and a 2ml sample of whole blood will be collected during their routine blood donation into an EDTA tube, gently mixed, and stored before testing [16].
Study Population
The research will focus on individuals aged between 18 and 65 who have met the blood bank's specific criteria for blood donation and have been accepted for donation at the Princess Christian Maternity Hospital Blood Bank [17].
Study Size
All blood donors came to donate blood at PCMH/ODCH blood bank. All positive sickling samples have to undergo a Hb Electrophoresis test at Ramsy Medical laboratory.
Fischer’s formula:

Where;
n= sample size
Z= normal deviation at the desired confidence interval
P= proportion of the population with the desired characteristics
Q= proportion of the population without the desired characteristics
I = degree of precision/ margin of error
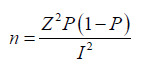
Z= 95% = 1.96 [25]
P= 0.5
Q= 1-P
I= 6.93%= 0.0693

Sample Collection, Transportation, Safety, and Storage & Maintenance Procedures
Sample Collection Procedure:
i. Tie a tourniquet on the arm to identify the skin area for sample collection.
ii. Clean the identified area with an alcohol swab to ensure proper disinfection.
iii. Perform venipuncture using a needle and syringe from the antecubital fossa in the arm or back of the hands.
iv. Transfer the collected blood sample into a 2-5ml EDTA tube.
v. Remove the tourniquet once the vein has been punctured.
vi. Place cotton wool on the puncture site and ask the person to stretch forth their hand.
vii. Discard the cotton wool once the blood stops flowing.
Sample Transportation:
i. Use a triple package system to transport samples from the PCMH/ODCH blood bank to the RAMSY Medical laboratory for analysis.
ii. Place the primary sample container into a leak-proof secondary container.
iii. Add absorbent material (e.g., gauze) in the secondary container to absorb any spillage.
iv. Put the sealed secondary container into a tertiary container for additional protection.
Safety:
1. Handling of Samples:
i. Treat all samples as potentially infectious.
ii. Wear appropriate personal protective equipment (PPE) when handling samples.
iii. Dispos of used PPE appropriately after sample collection.
iv. Follow standard operating procedures (SOPs) for sample handling.
2. Biohazard Disposal:
i. Dispose of biohazardous waste according to their specifications.
ii. Place biohazardous materials in designated biohazard bags.
iii. Dispose of sharps (e.g., needles) in sharps containers.
3. Documentation:
i. Follow SOPs for proper documentation of sample collection and handling.
ii. Ensure accurate and complete recording of relevant information.
Storage & Maintenance:
1. Reagent and Sample Storage:
i. Store reagents and samples in a refrigerator set between 2°C and 8°C.
ii. Place reagents and samples in their designated areas within the refrigerator.
iii. Label reagent bottles with the date of opening to track their usage.
2. Equipment Cleaning:
i. Clean equipment used for sample collection and analysis according to SOPs.
ii. Follow appropriate cleaning procedures to maintain equipment integrity and prevent contamination (Table 1).
Blood Grouping: ABO & Rh System
a) Principle: when red cells carrying one or both antigens are exposed to a corresponding antibody, they interact with each other to form visible clumps. It’s based on an agglutination reaction.
• Blood group A has the A antigen.
• Blood group B has the B antigen.
• Blood group AB has the A and B antigens.
• Blood group O lacks the A and B antigens.
Procedure:
1. Label a clean, dry tile with the letters A, B, AB, and D.
2. Place one drop of anti-A serum on the labeled area corresponding to A, one drop of anti-B serum on the labeled area corresponding to B, one drop of anti-AB serum on the labeled area corresponding to AB, and one drop of anti-D serum on the labeled area corresponding to D.
3. Add one drop of whole blood to each drop of the respective anti-serum.
4. Mix the cells and reagents evenly using a clean spreader or stick.
5. Tilt the tile and leave the test for 2 minutes at room temperature (approximately 25 degrees Celsius).
6. Gently rock the tile again.
7. Observe the tile for agglutination (clumping of cells) and record the results.
Interpretation: (Table 2).
Note:
Key
Agglutination: “+”
No agglutination: “-”
Quality Control: ABO and Rh control tests were conducted to ensure the reagents were functioning properly.
Precautions:
1. Ensure the anti-serum is brought to room temperature before use and promptly returned to a refrigerator set at 2-8 degrees Celsius after use.
2. Check that there are no particles present in the reagents.
3. Verify that the reagents are not expired.
4. Use clean and dry tiles or slides for the test.
5. Use different spreaders or spatula for mixing to prevent contamination.
Data Analysis
According to Table 3 above, out of 200 participants, 183(92%) of them are Male participants, and the remaining 17(9%) of the participants are Female. The cumulative percent can be interpreted as 92% of the participants being Male and 100% of the participants being either Female or Male participants. According to Table 4 above, out of 200 participants, 96(48%) of the age group are 18-25yrs old, and the remaining 73, 18, 10, and 3(37%, 9%, 5%, and 2%) of the participant’s age groups are 26-35yrs, 36-45yrs, 46-55yrs, and 56-69yrs old. The cumulative percent can be interpreted as 48% of the participants are 18-25 yrs old and 100% of the participants are either 26-35 yrs old or 36-45yrs, 46-55yrs, and 56-69yrs old. According to Table 5 above, out of 200 participants, 102(51%) of the participants belong to the blood group O+, and the remaining 47, 41, 6, and 4(24%, 21%, 3%, and 2%) of the participant’s blood groups are A+, B+, AB+, and O-. The cumulative percent can be interpreted as 51% of the participants are in the blood group O+ and 100% of the participants are either in the blood groups A+, B+, AB+, and O-. According to Table 6 above, out of 200 participants, 157(79%) of the participant’s test results came out Negative, and the remaining 43(22%) of the participant’s test results came out Positive. The cumulative percent can be interpreted as 79% of the participant’s test results being Negative and 100% of the participant’s test results being either Positive or Negative (Figures 1-5).
According to Table 7 above, out of 200 participants, 154(77%) of the participants belong to the Cellulose Acetate Paper HB Electrophoresis group AA, and the remaining 45, and 1 (23%, and 1%) of the participants Cellulose Acetate Paper HB Electrophoresis groups are AS and AC. The cumulative percent can be interpreted as 77% of the participants are in the Cellulose Acetate Paper HB Electrophoresis group AA and 100% of the participants are either in the Cellulose Acetate Paper HB Electrophoresis groups AS, and AC. According to Table 8 above, out of 200 observations, the minimum age group is 56-69yrs, and the maximum age group is 18-25yrs, with average age groups of 1.75 and a standard deviation of the age groups is 1.472. According to Table 9 above, the number of counts in each cell is the total number of concurrences for the intersection of the categories of the two variables. The total number of Male participants in the age group of 18-25yrs is 92(95.8%), which is different for the Female participants in the age group of 18-25yrs is 4(4.2%). The second row shows the total number of Male participants in the age group of 26-35yrs is 68(93.2%), which is different for the Female participants in the age group of 26-35yrs is 5(6.8%). The fourth row shows the total number of Male participants in the age group of 36-45yrs is 13(72.2%), which is different for the Female participants in the age group of 36-45yrs is 5(27.8%). The fifth row shows the total number of Male participants in the age group of 46-55yrs is 7(70.0%), which is different for the Female participants in the age group of 46-55yrs is 3(30.0%) (Figures 6-10).
Table 10: Show the Comparison of the Age groups of participants * Gender of Participants *Cellulose Acetate Paper HB Electrophoresis Crosstabulation.

The last row shows the total number of Male participants in the age group of 56-69yrs is 3(100.0%), and there is no Female for the age group of 56-69yrs. According to Table 10 above, the number of counts in each cell is the total number of concurrences for the intersection of the categories of the three variables. For The First row, the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 18-25yrs is 73(96.1%), which is different for the Female participants that are in the Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 18-25yrs is 3(3.9%). This row shows the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 26-35yrs is 55(91.7%), which is different for the Female participants in the Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 26-35yrs is 5(8.3%). This row shows the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 36-45yrs is 7(63.6%), which is different for the Female participants that are he Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 36-45yrs is 4(36.4%). This row shows the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 46-55yrs is 2(50.0%), which is different for the Female participants that are in the Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 46-55yrs is 2(50.0%) (Figures 11-15).
This row shows the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 56-69yrs is 3(100.0%), which is different for the Female participants that are in the Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 56-69yrs is 0(0.00%). For The second row, the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis Group AS in the age group of 18-25yrs is 19(95.0%), which is different for the Female participants that are in the Cellulose Acetate Paper HB Electrophoresis Group AS in the age group of 18-25yrs is 1(5.0%). This row shows the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis Group AS in the age group of 26-35yrs is 13(100.0%), which is different for the Female participants that are in the Cellulose Acetate Paper HB Electrophoresis Group AS in the age group of 26-35yrs is 0(0.00%). This row shows the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis Group AS in the age group of 36-45yrs is 5(83.3%), which is different for the Female participants that are in cellulose acetate Paper HB Electrophoresis Group AS in the age group of 36-45yrs is 1(16.7%). This row shows the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis Group AS in the age group of 46-55yrs is 5(83.3%), which is different for the Female participants that are in the Cellulose Acetate Paper HB Electrophoresis Group AS in the age group of 46-55yrs is 1(16.7%) (Figures 16-18).
For the last row, the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis Group AS in the age group of 36-45yrs is 1(100.0%%), which is different for the Female participants that are in the Cellulose Acetate Paper HB Electrophoresis Group AS in the age group of 36-45yrs is 0(0.00%). According to Table 11 above, the number of counts in each cell is the total number of concurrences for the intersection of the categories of the three variables. For The First row, the total number of Male participants that are in Blood Group O- in the age group of 26-35yrs is 1(100.0%), which is different for the Female participants that are in Blood Group O- in the age group of 26-35yrs is 0(0.00%). This row shows the total number of Male participants that are in the Blood Group O- in the age group of 36-45yrs is 1(50.0%), which is different for the Female participants that are in the Blood Group O- in the age group of 36-45yrs is 1(50.0%). This row shows the total number of Male participants that are in Blood Group O- in the age group of 46-55yrs is 0(0.00%), which is different for the Female participants that are in Blood Group O- in the age group of 46-55yrs is 1(100.0%). For The second row, the total number of Male participants that are in Blood Group A+ in the age group of 18-25yrs is 26(100.0%), which is different for the Female participants that are in Blood Group A+ in the age group of 18-25yrs is 0(0.00%) (Figures 19-22).
Table 11: Show the Comparison of Age groups of participants* Gender of All Participantss *Participants’ Blood Groups Crosstabulation.

This row shows the total number of Male participants that are in the Blood Group A+ in the age group of 26-35yrs is 12(80.0%), which is different for the Female participants that are in the Blood Group A+ in the age group of 26-35yrs is 3(20.0%). This row shows the total number of Male participants that are in the Blood Group A+ in the age group of 36-45yrs is 1(33.3%), which is different for the Female participants that are in the Blood Group A+ in the age group of 36-45yrs is 2(66.7%). This row shows the total number of Male participants that are in the Blood Group A+ in the age group of 46-55yrs is 2(100.0%), which is different for the Female participants that are in the Blood Group A+ in the age group of 46-55yrs is 0(0%). For the last row, the total number of Male participants that are in the Blood Group A+ in the age group of 56-69yrs is 1(100.0%), which is different for the Female participants that are in the Blood Group A+ in the age group of 56-69yrs is 0(0%). For the third row, the total number of Male participants that are in Blood Group AB+ in the age group of 18-25yrs is 3(100.0%), which is different for the Female participants that are in Blood Group AB+ in the age group of 18-25yrs is 0(0%). This row shows the total number of Male participants that are in the Blood Group AB+ in the age group of 26-35yrs is 1(100.0%), which is different for the Female participants that are in the Blood Group AB+ in the age group of 26-35yrs is 0(0%) [18].
This row shows the total number of Male participants that are in the Blood Group AB+ in the age group of 36-45yrs is 1(100.0%), which is different for the Female participants that are in the Blood Group AB+ in the age group of 36-45yrs is 0(0%). This row shows the total number of Male participants that are in the Blood Group AB+ in the age group of 46-55yrs is 1(100.0%), which is different for the Female participants that are in the Blood Group AB+ in the age group of 46-55yrs is 0(0%). For The last row, the total number of Male participants that are in Blood Group O+ in the age group of 18-25yrs is 43(97.7%), which is different for the Female participants that are in Blood Group O+ in the age group of 18-25yrs is 1(2.3%). This row shows the total number of Male participants that are in the Blood Group O+ in the age group of 26-35yrs is 41(95.3%), which is different for the Female participants that are in the Blood Group O+ in the age group of 26-35yrs is 2(4.7%). This row shows the total number of Male participants that are in the Blood Group O+ in the age group of 36-45yrs is 8(88.9%), which is different for the Female participants that are in the Blood Group O+ in the age group of 36-45yrs is 1(11.1%). This row shows the total number of Male participants that are in the Blood Group O+ in the age group of 46-55yrs is 3(75.0%), which is different for the Female participants that are in the Blood Group O+ in the age group of 46-55yrs is 1(25.0%). For the last row, the total number of Male participants that are in the Blood Group O+ in the age group of 56-69yrs is 2(100.0%), which is different for the Female participants that are in the Blood Group O+ in the age group of 56-69yrs is 0(0%) [19].
Table 12: Show the Comparison of Age groups of participants* Gender for All participants *Sodium Metabisulphite Wet Prep Crosstabulation.

According to Table 12 above, the number of counts in each cell is the total number of concurrences for the intersection of the categories of the three variables. For The First row, the total number of Male participants whose test results are positive for sodium Metabisulphite wet prep in the age group of 18-25yrs is 17(94.4%), which is different for the Female participants whose test results are positive for sodium Metabisulphite wet prep in the age group of 18-25yrs is 1(5.6%). This row shows the total number of Male participants whose test results are positive for sodium Metabisulphite wet prep in the age group of 26-35yrs is 13(92.9%), which is different for the Female participants whose test results are positive for sodium Metabisulphite wet prep in the age group of 26-35yrs is 1(7.1%). This row shows the total number of Male participants whose test results are positive for sodium Metabisulphite wet prep in the age group of 36-45yrs is 4(66.7%), which is different for the Female participants whose test results are positive for sodium Metabisulphite wet prep in the age group of 36-45yrs is 2(33.3%). This row shows the total number of Male participants whose test results are positive for sodium Metabisulphite wet prep in the age group of 46-55yrs is 5(100.0%), which is different for the Female participants whose test results are positive for sodium Metabisulphite wet prep in the age group of 46-55yrs is 0(0%) (Figure 23).
For the Second row, the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 18-25yrs is 75(96.2%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 18-25yrs is 3(3.8%). This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 26-35yrs is 55(93.2%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 26-35yrs is 4(6.8%). This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 36-45yrs is 9(75.0%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 36-45yrs is 3(25.0%). This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 46-55yrs is 2(40.0%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 46-55yrs is 3(60.0%). This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 56-69yrs is 3(100.0%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 56-69yrs is 0(0%) (Figure 24).
Table 13: Show the Comparison of ABO and Rh Blood Groups *Gender for All Participant *Sodium Metabisulphite Wet Prep Crosstabulation.

According to Table 13 above, the number of counts in each cell is the total number of concurrences for the intersection of the categories of the three variables. For The First row, the total number of Male participants whose test results are positive for sodium Metabisulphite wet prep in blood group A+ is 9(81.8%), which is different for the Female participants whose test results are positive for sodium Metabisulphite wet prep in blood group A+ is 2(18.2%). This row shows the total number of Male participants whose test results are positive for sodium Metabisulphite wet prep in blood group B+ is 10(83.3%), which is different for the Female participants whose test results are positive for sodium Metabisulphite wet prep in the blood group B+ is 2(16.7%). This row shows the total number of Male participants whose test results are positive for sodium Metabisulphite wet prep in the blood group O+ is 20(100.0%), which is different for the Female participants whose test results are positive for sodium Metabisulphite wet prep in the blood group O+ is 0(0%). For the Second row, the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the blood group O- is 2(50.0%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the blood group O- is 2(50.0%).
Table 14: Show the Comparison of Cellulose Acetate Paper HB Electrophoresis *Gender for All Participant *Sodium Metabisulphite Wet Prep Crosstabulation.

This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the A+ is 33(91.7%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the blood group A+ is 3(8.3%). This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the blood group B+ is 26(89.7%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the blood group B+ is 3(10.3%). This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the blood group AB+ is 6(100.0%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the blood group AB+ is 0(0%). This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the blood group is 77(93.9%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the blood group is 5(6.1%). According to Table 14 above, the number of counts in each cell is the total number of concurrences for the intersection of the categories of the three variables. For The First row, the total number of Male participants whose test results are positive for sodium Metabisulphite wet prep in group AA is 0(0%), which is different for the Female participants whose test results are positive for sodium Metabisulphite wet prep in group AA is 2(100.0%).
Table 15: Show the Comparison of ABO and Rh Blood Groups *Sodium Metabisulphite Wet Prep *Cellulose Acetate Paper HB Electrophoresis Crosstabulation.
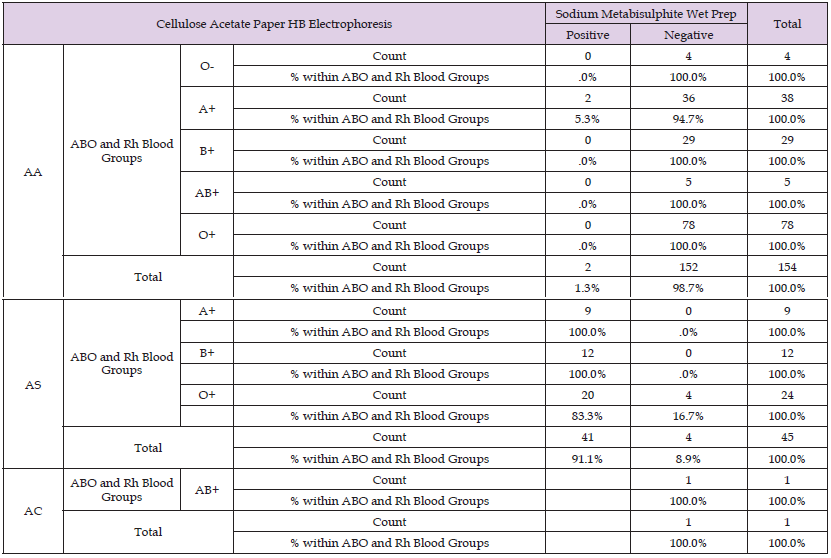
This row shows the total number of Male participants whose test results are positive for sodium Metabisulphite wet prep in group AS is 39(95.1%), which is different for the Female participants whose test results are positive for sodium Metabisulphite wet prep in group AS is 2(4.9%). For the Second row, the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in group AA is 140(92.1%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in group AA is 12(7.9%). This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in group AS is 3(75.0%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in group AS is 1(25.0%). This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in group AC is 1(100.0%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in group AC is 0(0%). According to Table 15 above, the number of counts in each cell is the total number of concurrences for the intersection of the categories of the three variables.
For The First row, the total number of participants who are positive that are in Group AA of Blood group O- is 0(0.0%), which is different for participants who are negative that are in Group AA of Blood group O- is 4(100.0%), the total number of participants who are positive that are in Group AA of Blood group A+ is 2(5.3%), which is different for participants who are negative that are in Group AA of Blood group A+ is 36(94.7%), the total number of participants who are positive that are in Group AA of Blood group B+ is 0(0.0%), which is different for participants who are negative that are in Group AA of Blood group B+ is 29(100.0%), the total number of participants who are positive that are in Group AA of Blood group AB+ is 0(0.0%), which is different for participants who are negative that are in Group AA of Blood group AB+ is 5(100.0%), the total number of participants who are positive that are in Group AA of Blood group O+ is 0(0.0%), which is different for participants who are negative that are in Group AA of Blood group O+ is 78(100.0%). For The Second row, the total number of participants who are positive that are in Group AS of A+ is 9(100.0%), which is different for participants who are negative that are in Group AS of Blood group A+ is 0(0.0%), the total number of participants who are positive that are in Group AS of Blood group B+ is 12(100.0%), which is different for participants who are negative that are in Group AS of Blood group B+ is 0(0.0%), the total number of participants who are positive that are in Group AS of Blood group O+ is 20(83.3%), which is different for participants who are negative that are in Group AS of Blood group O+ is 4(16.7%).
Table 16: Show The Comparism of The Age Groups Of respondents *Cellulose Acetate Paper HB Electrophoresis Crosstabulation of All Participants.

For The Last row, the total number of participants who are positive that are in Group AC of Blood group AB+ is 0(0.0%), which is different for participants who are negative that are in Group AC of Blood group AB+ is 1(100.0%), According to Table 16 above, the number of counts in each cell is the total number of concurrences for the intersection of the categories of the two variables. The total number of participants in group AA in the age group of 18-25yrs is 76(79.2%), which is different for the participants In AS group in the age group of 18-25yrs is 20(20.8%), which is different for the participants In AC group in the age group of 18-25yrs is 0(0.0%). For the second row the total number of participants in group AA in the age group of 26-35yrs is 60(82.2%), which is different for the participants In AS group in the age group of 26-35yrs is 13(17.8%), which is different for the participants In AC group in the age group of 26-35yrs is 0(0.0%). For the third row the total number of participants in group AA in the age group of 36-45yrs is 11(61.1%), which is different for the participants In AS group in the age group of 36-45yrs is 6(33.3%), which is different for the participants In AC group in the age group of 36-45yrs is 1(5.6%). For the fourth row the total number of participants in group AA in the age group of 46-55yrs is 4(40.0%), which is different for the participants In AS group in the age group of 46-55yrs is 6(60.0%), which is different for the participants In AC group in the age group of 46-55yrs is 0(0.0%).
For the last row the total number of participants in group AA in the age group of 56-69yrs is 3(100.0%), which is different for the participants In AS group in the age group of 56-69yrs is 0(0.0%), which is different for the participants In AC group in the age group of 56-69yrs is 0(0.0%).
Conclusion
Summary of Research: This study focusses to determine the rate of HBS among blood donors in PCMH and ODCH Blood Bank in the selected hospitals were this study was conducted. Based on the secondary data collected from the two hospitals, 6 variables were analysed and were not taken for granted. Studies in the area were reduced to the period of already admitted patient’s records in the two hospitals. Short term evaluation of data collected within the short term interval. Research was undertaken on adult’s age 18yrs to 69yrs in two selected hospitals. The cases study showed rate of HBS among blood donors on patients with different initial levels. Rate of HBS among blood donors on the patients was observed using secondary data using parameters (6 variables) relevant to the study.
Discussion and Interpretation of Findings:
Frequency Analysis: According to Table 3 above, out of 200 participants, 183(92%) of them are Male participants, and the remaining 17(9%) of the participants are Female. The cumulative percent can be interpreted as 92% of the participants being Male and 100% of the participants being either Female or Male participants. According to Table 4 above, out of 200 participants, 96(48%) of the age group are 18-25yrs old, and the remaining 73, 18, 10, and 3(37%, 9%, 5%, and 2%) of the participant’s age groups are 26-35yrs, 36-45yrs, 46-55yrs, and 56-69yrs old. The cumulative percent can be interpreted as 48% of the participants are 18-25 yrs old and 100% of the participants are either 26-35 yrs old or 36-45yrs, 46-55yrs, and 56-69yrs old.
According to Table 5 above, out of 200 participants, 102(51%) of the participants belong to the blood group O+, and the remaining 47, 41, 6, and 4(24%, 21%, 3%, and 2%) of the participant’s blood groups are A+, B+, AB+, and O-. The cumulative percent can be interpreted as 51% of the participants are in the blood group O+ and 100% of the participants are either in the blood groups A+, B+, AB+, and O-. According to Table 6 above, out of 200 participants, 157(79%) of the participant’s test results came out Negative, and the remaining 43(22%) of the participant’s test results came out Positive. The cumulative percent can be interpreted as 79% of the participant’s test results being Negative and 100% of the participant’s test results being either Positive or Negative.
According to Table 7 above, out of 200 participants, 154(77%) of the participants belong to the Cellulose Acetate Paper HB Electrophoresis group AA, and the remaining 45, and 1 (23%, and 1%) of the participant’s Cellulose Acetate Paper HB Electrophoresis groups are AS and AC. The cumulative percent can be interpreted as 77% of the participants are in the Cellulose Acetate Paper HB Electrophoresis group AA and 100% of the participants are either in the Cellulose Acetate Paper HB Electrophoresis groups AS, and AC.
Descriptive Analysis: According to Table 8 above, out of 200 observations, the minimum age group is 56-69yrs, and the maximum age group is 18-25yrs, with average age groups of 1.75 and a standard deviation of the age groups is 1.472.
Cross-tabulation Analysis: According to Table 9 above, the number of counts in each cell is the total number of concurrences for the intersection of the categories of the two variables. The total number of Male participants in the age group of 18-25yrs is 92(95.8%), which is different for the Female participants in the age group of 18-25yrs is 4(4.2%). The second row shows the total number of Male participants in the age group of 26-35yrs is 68(93.2%), which is different for the Female participants in the age group of 26-35yrs is 5(6.8%). The fourth row shows the total number of Male participants in the age group of 36-45yrs is 13(72.2%), which is different for the Female participants in the age group of 36-45yrs is 5(27.8%). The fifth row shows the total number of Male participants in the age group of 46-55yrs is 7(70.0%), which is different for the Female participants in the age group of 46-55yrs is 3(30.0%). The last row shows the total number of Male participants in the age group of 56-69yrs is 3(100.0%), and there is no Female for the age group of 56-69yrs. According to Table 10 above, the number of counts in each cell is the total number of concurrences for the intersection of the categories of the three variables. For The First row, the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 18-25yrs is 73(96.1%), which is different for the Female participants that are in the Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 18-25yrs is 3(3.9%).
This row shows the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 26-35yrs is 55(91.7%), which is different for the Female participants in the Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 26-35yrs is 5(8.3%). This row shows the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 36-45yrs is 7(63.6%), which is different for the Female participants that are he Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 36-45yrs is 4(36.4%). This row shows the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 46-55yrs is 2(50.0%), which is different for the Female participants that are in the Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 46-55yrs is 2(50.0%). This row shows the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 56-69yrs is 3(100.0%), which is different for the Female participants that are in the Cellulose Acetate Paper HB Electrophoresis group AA in the age group of 56-69yrs is 0(0.00%). For The second row, the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis Group AS in the age group of 18-25yrs is 19(95.0%), which is different for the Female participants that are in the Cellulose Acetate Paper HB Electrophoresis Group AS in the age group of 18-25yrs is 1(5.0%).
This row shows the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis Group AS in the age group of 26-35yrs is 13(100.0%), which is different for the Female participants that are in the Cellulose Acetate Paper HB Electrophoresis Group AS in the age group of 26-35yrs is 0(0.00%). This row shows the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis Group AS in the age group of 36-45yrs is 5(83.3%), which is different for the Female participants that are in cellulose acetate Paper HB Electrophoresis Group AS in the age group of 36-45yrs is 1(16.7%). This row shows the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis Group AS in the age group of 46-55yrs is 5(83.3%), which is different for the Female participants that are in the Cellulose Acetate Paper HB Electrophoresis Group AS in the age group of 46-55yrs is 1(16.7%). For the last row, the total number of Male participants that are in the Cellulose Acetate Paper HB Electrophoresis Group AS in the age group of 36-45yrs is 1(100.0%%), which is different for the Female participants that are in the Cellulose Acetate Paper HB Electrophoresis Group AS in the age group of 36-45yrs is 0(0.00%). According to Table 11 above, the number of counts in each cell is the total number of concurrences for the intersection of the categories of the three variables.
For The First row, the total number of Male participants that are in Blood Group O- in the age group of 26-35yrs is 1(100.0%), which is different for the Female participants that are in Blood Group O- in the age group of 26-35yrs is 0(0.00%). This row shows the total number of Male participants that are in the Blood Group O- in the age group of 36-45yrs is 1(50.0%), which is different for the Female participants that are in the Blood Group O- in the age group of 36-45yrs is 1(50.0%). This row shows the total number of Male participants that are in Blood Group O- in the age group of 46-55yrs is 0(0.00%), which is different for the Female participants that are in Blood Group O- in the age group of 46-55yrs is 1(100.0%). For The second row, the total number of Male participants that are in Blood Group A+ in the age group of 18-25yrs is 26(100.0%), which is different for the Female participants that are in Blood Group A+ in the age group of 18-25yrs is 0(0.00%). This row shows the total number of Male participants that are in the Blood Group A+ in the age group of 26-35yrs is 12(80.0%), which is different for the Female participants that are in the Blood Group A+ in the age group of 26-35yrs is 3(20.0%). This row shows the total number of Male participants that are in the Blood Group A+ in the age group of 36-45yrs is 1(33.3%), which is different for the Female participants that are in the Blood Group A+ in the age group of 36-45yrs is 2(66.7%).
This row shows the total number of Male participants that are in the Blood Group A+ in the age group of 46-55yrs is 2(100.0%), which is different for the Female participants that are in the Blood Group A+ in the age group of 46-55yrs is 0(0%). For the last row, the total number of Male participants that are in the Blood Group A+ in the age group of 56-69yrs is 1(100.0%), which is different for the Female participants that are in the Blood Group A+ in the age group of 56-69yrs is 0(0%). For the third row, the total number of Male participants that are in Blood Group AB+ in the age group of 18-25yrs is 3(100.0%), which is different for the Female participants that are in Blood Group AB+ in the age group of 18-25yrs is 0(0%). This row shows the total number of Male participants that are in the Blood Group AB+ in the age group of 26-35yrs is 1(100.0%), which is different for the Female participants that are in the Blood Group AB+ in the age group of 26-35yrs is 0(0%). This row shows the total number of Male participants that are in the Blood Group AB+ in the age group of 36-45yrs is 1(100.0%), which is different for the Female participants that are in the Blood Group AB+ in the age group of 36-45yrs is 0(0%). This row shows the total number of Male participants that are in the Blood Group AB+ in the age group of 46-55yrs is 1(100.0%), which is different for the Female participants that are in the Blood Group AB+ in the age group of 46-55yrs is 0(0%).
For The last row, the total number of Male participants that are in Blood Group O+ in the age group of 18-25yrs is 43(97.7%), which is different for the Female participants that are in Blood Group O+ in the age group of 18-25yrs is 1(2.3%). This row shows the total number of Male participants that are in the Blood Group O+ in the age group of 26-35yrs is 41(95.3%), which is different for the Female participants that are in the Blood Group O+ in the age group of 26-35yrs is 2(4.7%). This row shows the total number of Male participants that are in the Blood Group O+ in the age group of 36-45yrs is 8(88.9%), which is different for the Female participants that are in the Blood Group O+ in the age group of 36-45yrs is 1(11.1%). This row shows the total number of Male participants that are in the Blood Group O+ in the age group of 46-55yrs is 3(75.0%), which is different for the Female participants that are in the Blood Group O+ in the age group of 46-55yrs is 1(25.0%). For the last row, the total number of Male participants that are in the Blood Group O+ in the age group of 56-69yrs is 2(100.0%), which is different for the Female participants that are in the Blood Group O+ in the age group of 56-69yrs is 0(0%). According to Table 12 above, the number of counts in each cell is the total number of concurrences for the intersection of the categories of the three variables. For The First row, the total number of Male participants whose test results are positive for sodium Metabisulphite wet prep in the age group of 18-25yrs is 17(94.4%), which is different for the Female participants whose test results are positive for sodium Metabisulphite wet prep in the age group of 18-25yrs is 1(5.6%).
This row shows the total number of Male participants whose test results are positive for sodium Metabisulphite wet prep in the age group of 26-35yrs is 13(92.9%), which is different for the Female participants whose test results are positive for sodium Metabisulphite wet prep in the age group of 26-35yrs is 1(7.1%). This row shows the total number of Male participants whose test results are positive for sodium Metabisulphite wet prep in the age group of 36-45yrs is 4(66.7%), which is different for the Female participants whose test results are positive for sodium Metabisulphite wet prep in the age group of 36-45yrs is 2(33.3%). This row shows the total number of Male participants whose test results are positive for sodium Metabisulphite wet prep in the age group of 46-55yrs is 5(100.0%), which is different for the Female participants whose test results are positive for sodium Metabisulphite wet prep in the age group of 46-55yrs is 0(0%). For the Second row, the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 18-25yrs is 75(96.2%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 18-25yrs is 3(3.8%). This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 26-35yrs is 55(93.2%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 26-35yrs is 4(6.8%).
This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 36-45yrs is 9(75.0%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 36-45yrs is 3(25.0%). This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 46-55yrs is 2(40.0%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 46-55yrs is 3(60.0%). This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 56-69yrs is 3(100.0%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the age group of 56-69yrs is 0(0%). According to Table 13 above, the number of counts in each cell is the total number of concurrences for the intersection of the categories of the three variables. For The First row, the total number of Male participants whose test results are positive for sodium Metabisulphite wet prep in blood group A+ is 9(81.8%), which is different for the Female participants whose test results are positive for sodium Metabisulphite wet prep in blood group A+ is 2(18.2%).
This row shows the total number of Male participants whose test results are positive for sodium Metabisulphite wet prep in blood group B+ is 10(83.3%), which is different for the Female participants whose test results are positive for sodium Metabisulphite wet prep in the blood group B+ is 2(16.7%). This row shows the total number of Male participants whose test results are positive for sodium Metabisulphite wet prep in the blood group O+ is 20(100.0%), which is different for the Female participants whose test results are positive for sodium Metabisulphite wet prep in the blood group O+ is 0(0%). For the Second row, the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the blood group O- is 2(50.0%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the blood group O- is 2(50.0%). This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the A+ is 33(91.7%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the blood group A+ is 3(8.3%). This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the blood group B+ is 26(89.7%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the blood group B+ is 3(10.3%).
This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the blood group AB+ is 6(100.0%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the blood group AB+ is 0(0%). This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in the blood group is 77(93.9%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in the blood group is 5(6.1%). According to Table 14 above, the number of counts in each cell is the total number of concurrences for the intersection of the categories of the three variables. For The First row, the total number of Male participants whose test results are positive for sodium Metabisulphite wet prep in group AA is 0(0%), which is different for the Female participants whose test results are positive for sodium Metabisulphite wet prep in group AA is 2(100.0%). This row shows the total number of Male participants whose test results are positive for sodium Metabisulphite wet prep in group AS is 39(95.1%), which is different for the Female participants whose test results are positive for sodium Metabisulphite wet prep in group AS is 2(4.9%). For the Second row, the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in group AA is 140(92.1%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in group AA is 12(7.9%).
This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in group AS is 3(75.0%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in group AS is 1(25.0%). This row shows the total number of Male participants whose test results are negative for sodium Metabisulphite wet prep in group AC is 1(100.0%), which is different for the Female participants whose test results are negative for sodium Metabisulphite wet prep in group AC is 0(0%). According to Table 15 above, the number of counts in each cell is the total number of concurrences for the intersection of the categories of the three variables. For The First row, the total number of participants who are positive that are in Group AA of Blood group O- is 0(0.0%), which is different for participants who are negative that are in Group AA of Blood group O- is 4(100.0%), the total number of participants who are positive that are in Group AA of Blood group A+ is 2(5.3%), which is different for participants who are negative that are in Group AA of Blood group A+ is 36(94.7%), the total number of participants who are positive that are in Group AA of Blood group B+ is 0(0.0%), which is different for participants who are negative that are in Group AA of Blood group B+ is 29(100.0%), the total number of participants who are positive that are in Group AA of Blood group AB+ is 0(0.0%), which is different for participants who are negative that are in Group AA of Blood group AB+ is 5(100.0%), the total number of participants who are positive that are in Group AA of Blood group O+ is 0(0.0%), which is different for participants who are negative that are in Group AA of Blood group O+ is 78(100.0%).
For The Second row, the total number of participants who are positive that are in Group AS of A+ is 9(100.0%), which is different for participants who are negative that are in Group AS of Blood group A+ is 0(0.0%), the total number of participants who are positive that are in Group AS of Blood group B+ is 12(100.0%), which is different for participants who are negative that are in Group AS of Blood group B+ is 0(0.0%), the total number of participants who are positive that are in Group AS of Blood group O+ is 20(83.3%), which is different for participants who are negative that are in Group AS of Blood group O+ is 4(16.7%). For The Last row, the total number of participants who are positive that are in Group AC of Blood group AB+ is 0(0.0%), which is different for participants who are negative that are in Group AC of Blood group AB+ is 1(100.0%). According to Table 16 above, the number of counts in each cell is the total number of concurrences for the intersection of the categories of the two variables. The total number of participants in group AA in the age group of 18-25yrs is 76(79.2%), which is different for the participants In AS group in the age group of 18-25yrs is 20(20.8%), which is different for the participants In AC group in the age group of 18-25yrs is 0(0.0%). For the second row the total number of participants in group AA in the age group of 26-35yrs is 60(82.2%), which is different for the participants In AS group in the age group of 26-35yrs is 13(17.8%), which is different for the participants In AC group in the age group of 26-35yrs is 0(0.0%).
For the third row the total number of participants in group AA in the age group of 36-45yrs is 11(61.1%), which is different for the participants In AS group in the age group of 36-45yrs is 6(33.3%), which is different for the participants In AC group in the age group of 36-45yrs is 1(5.6%). For the fourth row the total number of participants in group AA in the age group of 46-55yrs is 4(40.0%), which is different for the participants In AS group in the age group of 46-55yrs is 6(60.0%), which is different for the participants In AC group in the age group of 46-55yrs is 0(0.0%). For the last row the total number of participants in group AA in the age group of 56-69yrs is 3(100.0%), which is different for the participants In AS group in the age group of 56-69yrs is 0(0.0%), which is different for the participants In AC group in the age group of 56-69yrs is 0(0.0%).
Based on the findings of this study, the following recommendations are proposed:
1. Blood services should implement screening for sickle cell in all potential blood donors.
2. Confirmation of sickle cell status using Hb electrophoresis should be conducted for suspected individuals to determine their genotype accurately and identify any other abnormal haemoglobin genes. Staff should receive proper training in using Hb electrophoresis equipment and reporting results.
3. The necessary tests for sickle cell should be available in all renowned/teaching hospital laboratories and blood banks in the country.
4. Sickle cell testing should be conducted using both the wet prep method and Hb electrophoresis to minimize the occurrence of false positive or false negative results.
5. Counselling sessions should be conducted in schools, universities, and through radio and television programs to increase awareness of sickle cell prevalence and educate individuals on prevention and management of sickle cell trait and disease.


