Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Iftekhar Hossain Chowdhury1*, Yeasmin Jahan Afroze2, Shahrin Sultana3, Farjana Haque Mitu4, Shirin Aktar5 and Kamrun Nahar1
Received: November 09, 2023; Published: December 07, 2023
*Corresponding author: Iftekhar Hossain Chowdhury, Department of Pharmacology, Mugda Medical College and Hospital, Dhaka-1214, Bangladesh
DOI: 10.26717/BJSTR.2023.54.008487
Objective: The cornerstone of treating cancer-related anorexia-cachexia in patients with advanced cancer
is routine anorexia-cachexia assessment. There isn’t yet a validated instrument to evaluate anorexia among
Bengali-speaking people who have terminal illnesses. The study’s objectives are to translate the Functional
Assessment of Anorexia Cachexia Therapy (Anorexia/Cachexia Subscale) Version 4 questionnaire into Bengali
and evaluate it while making cultural adaptations.
Methods: There were two stages of the investigation. Four translators worked independently to translate
the Functional Assessment of Anorexia Cachexia Therapy (Anorexia/Cachexia Subscale) questionnaire from
English into Bengali, both forward and backward. The tool’s Bengali version was finalized during pre-testing
and cognitive debriefing, following reviews from an expert committee. Eighty patients from the clinical
oncology department of Bangabandhu Sheikh Mujib Medical University and NICRH, Dhaka, Bangladesh
participated in the final validation. In the last stage of validation, reliability (internal consistency) and validity
(content, face, and construct validity) were evaluated.
Result: Every participant answered every item. Thirty percent of participants struggled with three problems,
whereas seventy percent of participants understood every question completely. The questionnaire’s face
and content validity were deemed satisfactory by the expert committee. High dependability was also seen in
the Bengali version (α=0.84). The Functional Assessment of Anorexia Cachexia Therapy (Anorexia/Cachexia
Subscale) principal component analysis distribution of varimax rotation ranged from 0.53 to 0.85 in Bengali.
Conclusion: The Bengali version of the FAACT ACS was shown to be valid, relible, and culturally appropriate
for the Bengalis peaking people. As a result, it can be used in clinical and research settings to assess symptoms
and monitor treatment responses of patients suffering from advanced cancer in Bengali-speaking nations.
Keywords: FAACT ACS Bengali; Symptom Assessment; Bengali; Advanced Cancer; Bangladesh
Abbreviations: QoL: Quality of Life; FACT: Functional Assessment of Cancer Therapy; LCS: Lung Cancer Subscale; FAACT: Functional Assessment of Anorexia Cachexia Therapy
Patients with advanced cancer often experience anorexia (i.e., the subjective sense of poor appetite) and cachexia (i.e., the physiologic state of muscle catabolism and weight loss) [1]. These often occur together and constitute the “cancer related anorexiacachexia ” or CRAC [2,3]. To promote standardization, a 2011 international consensus defined CRAC, as “weight loss greater than 5 %, or weight loss greater than 2 % in individuals already showing depletion according to current bodyweight and height (body-mass index<20KM/M) or skeletal muscle mass (sarcopenia) [4]. We have previously shown that when using these criteria, patients with overt CRAC suffer a significant burden of symptoms, marked impairments in quality of life (QoL) [5], and shorter overall survival [6]. The measurement of QoL impairments related to CRAC is therefore important both in clinical practice and in research efforts [7] that seek to improve patients’ lived experience with advanced cancer. The Functional Assessment of Cancer Therapy (FACT) is a set of measures designed to assess health related QoL and is one of the most wellvalidated instruments established for use exclusively among cancer patients [8]. The FACT family is built around the core instrument FACT-General (FACT-G) and its four subscales: physical well-being, emotional well-being, functional well-being, and social well-being. The FACT-G is frequently paired with extra subscales that incorporate additional items relevant to certain therapeutic situations.
There is a “lung cancer subscale” (LCS) for patients with lung cancer, for example, which when combined with the FACT-G generates the FACT-L [9]. There is also anorexia and cachexia subscale (ACS) [10]. When paired with the FACT-G, the Functional Assessment of Anorexia Cachexia Therapy (FAACT) is formed. While both scales are said to cover crucial dimensions of health related QoL in cancer patients, some have questioned the FAACT scale’s utility and validity [11]. Because the FAACT and its anorexia-cachexia subscale (ACS) are relevant measures of QoL in patients with advanced cancer and CRAC, they were used in this study.
Functional Assessment of Anorexia Cachexia Therapy (Anorexia/ Cachexia Subscale) Version 4 questionnaire was devised in 2000 and consists of twelve questions, which each have five possible multiplechoice answers (not at all, little bit, somewhat, quite a bit, very much). The scoring system awards points from 0 to 4 for each question and is arranged so that the higher values equate to healthier scenarios. Each patient’s response applies to the previous week and a total score of up to 48 is possible. In this regard, we must mention that the FAACT ACS questionnaire is widely used in the clinical trials of CRAC, and its improvement is considered as a significant therapeutic potential.
Translation Procedure
The Beaton et al.7 criteria were followed during the English to Bengali translation procedure. Two separate translators who spoke Bengali completed the forward translation; one knew about the scale’s notion (T1), while the other did not (T2). Two forward translators were involved in the project: one had a medical background (T1), while the other was a non-medical expert in English literature (T2). A third, impartial party then integrated the two versions to create a combined one (Ts). Since the Ts version included practically all local customs, habits, and word usage, only a few alterations were needed. Two separate, highly proficient English translators (BT1 and BT2) back translated the questionnaire from its Ts version into English. Both of them had a strong background in English language and were not in the medical field. The tool’s design was concealed from these two back translators to reduce information bias and extract unexpected meanings from the translated questionnaire’s items. Uncertain language in the translations was accentuated by the back-translation procedure. Then an expert committee was formed with two experts from the Department of Chemistry, Dhaka College. Both were trained in the English version of FAACT ACS. The expert committee and all translators reviewed and compared all the translations and the original FAACT ACS in July 2022. They verified the semantic, idiomatic, experiential, and conceptual equivalence between the English and Bengali versions. After reaching a consensus among all the members of the committee, the final Bengali version was made for pre-testing (Table 1).
Pilot-Testing
Thirty postgraduate medical students at Bangabandhu Sheikh Mujib Medical University were given the pre-test for the final Bengali version of the FAACT ACS. The participants were asked to explain their understanding of each question and how they would respond if the condition applied to them. In August 2022, ten Bengalispeaking patients were hospitalized to the oncology department of NICRH, Dhaka, for cognitive debriefing. Following this, the pre-tested questionnaire was administered to them. Their information was not utilized for the primary study, merely for this process. When the questionnaire was being administered, the patients were questioned about any terms that they found challenging, perplexing, or disturbing. For words and phrases that were challenging or unclear, substitute words and succinct explanations were provided. The final version of the questionnaire was authorized following the expert committee’s examination and approval.
Setting and Participants for the Final Validation Phase
For the last validation stage, we included eighty patients. 80 responders in total were contacted to discuss a 10% dropout rate. Thus, 80 was the final sample size. The study comprised patients who could read and understand Bengali and who had advanced cancer and were hospitalized to the clinical oncology department of BSMMU and NICRH. The patients were aged approximately 18 years. The study excluded patients with psychotic illnesses or any form of cognitive impairment.
Data Collection
The period of data collecting was October 2022–December 2022. A sequential sampling technique was used to collect the samples. It was requested that eligible patients complete the questionnaire on their own. When necessary, the primary investigator provided them with succinct explanations of each item in case there was any doubt about it. Each questionnaire took five to ten minutes to complete.
Data Analysis
The face validity was assessed during the routine translation procedure. Content validity was verified throughout back-translation, standard translation, expert committee evaluation, and literature reviews. Construct validity was investigated using factor analysis along with principal component analysis and varimax rotation (Figure 1).
Ethical Consideration
The Department of Oncology, BSMMU, and NICR&H gave their consent. After reviewing the protocol, the BSMMU institutional review board (IRB) issued a clearance letter memo with the date BSMMU/2022/4293 on April 25. All eligible patients were made aware of the study’s goals and the intervention. The participants’ privacy was scrupulously protected. The participant’s private information, including name, age, sex, and other details, was kept private and utilized exclusively the study.
Validity Analysis
Face Validity: The translation and adaptation procedure also included the face validity methodology. Both the naive responders (uninformed translators) and the expert committee members agreed that the exam’s objective was clear: it should quantify the symptoms of patients who are nearing the end of their lives. 90% of the 30 students who took the pre-test comprehended every question, with the remaining 10% needing some explanation. Eighty percent of the patients fully understood seven questions during the cognitive debriefing, and twenty percent understood every question. The elements of the Bengali version were all connected to the subject of the measurement. The only two that needed a long explanation were “I have a good appetite” and “My general health is improving.” Both the measurement technique and the measure itself worked well for collecting and calculating the variables. The final instrument version was deemed by the expert panel to be a trustworthy tool for evaluating symptoms in the Bengali-speaking community.
Content Validity: Content validity of the final version was assessed by two experts: Item-level content validity index (I-CVI) was found to be 1 (Table 2) for each item and scalelevel content validity index (S-CVI) was therefore 1 by the averaging calculation method (Table 2).
Construct Validity: Most participants, who were almost equally divided between the sexes and aged between 30 and 60, took part in the final validation process. (Table 3) shows that most of them (59%) had completed secondary or higher secondary school. Using a principal component and varimax rotation, exploratory factor analysis was used to evaluate the final version’s construct validity. The observed Kaiser-Meyer-Olkin (KMO) score of 0.71 indicates that the factor analysis could be performed with the current data. The range of the FAACT-ACS Bengali varimax rotation distribution was 0.53 to 0.85. The item 8 (My family and friends are urging me to eat) received the highest score of 0.83. Item 11 (I feel stomachache) received the lowest score of 0.53 (Table 4). Out of the twelve items, just one component was taken out.
Table 4: Principal component analysis with the distribution of varimax rotation of FAACT ACS Bengali.
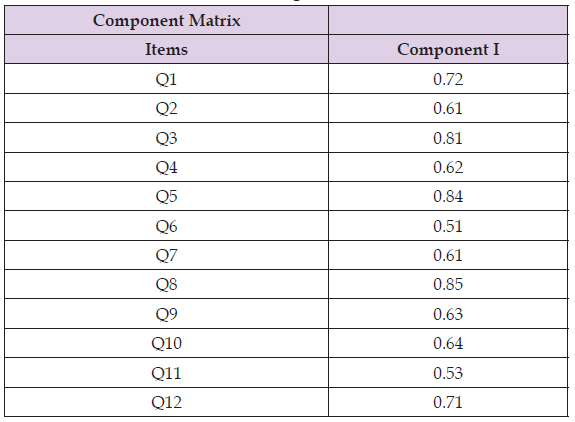
Note: Extraction method: principal component analysis I component extracted.
Reliability Analysis
The Cronbach’s alpha (α) value ⩾0.70 was considered as adequate and ⩾0.80 was considered as optimal. In our study, the Cronbach’s alpha (α) value for FAACT ACS Bengali was 0.84 (Table 5).
Translation and standardization of a scale are crucial components of cultural adaptation of a standard scale or instrument. Finding appropriate words in translated language sometimes becomes a great challenge for translators. In this study, we have translated and validated the FAACT ACS questionnaire which is one of the most important tools for assessment of anorexia cachexia in the field of advanced cancer patients. Several well-validated instruments in the FACT family are relevant to patients with advanced lung cancer; nevertheless, some issues have been raised concerning the validity of the FAACT scale and the ACS [11]. As a result, we investigated the scales’ cross-sectional validity. Our findings are as follows: encouraging-both scales show appropriate levels of Internal consistency, convergent validity, and divergent validity are all compatible with the other instruments in the FACT family. These findings, when supplemented with comprehensive validation of the FACT instruments outside of the current setting, indicate that give assurance regarding using the FAACT and the ACS to people suffering with advanced cancer. This tool’s face validity has likewise been determined to be highly consistent with the original version. The expert group determined that the version was correct for the symptom assessment of the Bengali-speaking population. Two factors (loss of appetite and well-being) required brief explanations during cognitive debriefing. Item number 1 is “I Have Good Appetite.” There are multiple synonyms and expressions for the term “I Have Good Appetite.”
Usually in Bengali when two positive words are used at a time, it becomes difficult to understand for the patient. Also, patients with lower educational status needed either further explanation or elaboration of the meaning of this phrase. Other studies showed that patients who evaluated the FAACT ACS stated that the term “Good Appetite” was a double positive and therefore led to confusion. Item number 12 is “My general health is improving” The term “general health” seems vague and non-specific to many of our participants. A further explanation was needed to clear their confusion. So, the expert committee decided to use a conceptual synonym for the meaning of general health. Where the meaning of the word “general health was not understood by the patients and hindered the overall rating of the instrument. The internal consistency of the Bengali version was 0.84, which denotes high reliability. It is as reliable as other language versions such as Spanish version (α=0.81), Arabic (α=0.85) [12,13]. During data collection, we had trouble determining whether the symptoms occurred “before or after receiving chemotherapy,” “at the time of admission,” or “at the time of data collection.” We wanted to emphasize that the term “now” solely refers to the symptoms that existed “at the time of data collection. One limitation of our study is that we were unable to do test-retest reliability because many of our patients were nearing the end of their lives and found it difficult to repeat this questionnaire. Another limitation is that we were unable to analyze the measure’s convergent validation because there are no alternative tool in Bengali for symptom evaluation in advance cancer patients.
Numerous studies have shown that stimulation of cellular ROS generation can lead to MAP kinase activation whereas antioxidants can inhibit activation. In the case of Mes 23.5 cells, Mn-induced activation of p38 MAP kinase preceded nuclear HIF-1α accumulation since pharmacologic inhibition of p38 MAP kinase blocked the expression of HIF-1α. Although the mechanism underlying HIF-1α regulation by p38 MAPK is unclear, it appears that p38 MAPK acts as a redox-sensitive factor to induce HIF-1α stabilization, followed by nuclear translocation to promote transcription of target genes. The functional result is a transfer of the redox signal to the nucleus to initiate transcription of pro-death gene products. As a transcription factor, HIF-1α regulates a series of target genes encoding proteins that promote cell survival [31]. On the other hand, HIF-1α can also participate in cell death by activating pro-death genes, including BNIP3, and Nix [32]. BNIP3 expression can be upregulated under both hypoxic and non-hypoxic conditions in cell lines derived from carcinomas, fibroblasts, and macrophages [32,33]. In the nervous system, BNIP3 functions as a pro-death factor in select brain regions following subarachnoid hemorrhage [34] or focal cerebral ischemia [35]. Recently, BNIP3 was linked to brain developmental apoptosis in which BNIP3 mRNA increased in parallel with developmental cell death in neonatal rat brains [36]. BNIP3 induction was shown to be regulated by HIF-1α and induces apoptotic cell death in many nonneuronal cell lines [Bruick [37,38]]. A similar induction of BNIP3 by Mn in a dopaminergic cell line was reported by us. Knockdown of BNIP3 by siRNA transfection protected cells from cytotoxicity, providing strong support for the role of BNIP3 in cell death [26]. Overall, these results, show that in Mn-induced cell death by upregulating HIF-1. Oxidative stress and p38 MAP kinase activation initiate upregulation of HIF-1α mediated signaling and its role in inducing the expression of cell death-promoting genes.
The Bengali version of the FAACT (anorexia/cachexia subscale) was shown to be valid, reliable, and culturally appropriate for the Bengalis peaking people. As a result, it can be used in clinical and research settings to assess symptoms and monitor treatment responses of patients suffering from advanced cancer in Bengalispeaking nations.
The authors gratefully acknowledge the contribution of Dr. Md Najmul Kabir Chowdhury, Associate Professor, Dhaka College, Dhaka; Dr. Selina Momtaz, Assistant Professor, Dhaka College, Dhaka and Khairul Alam, Assistant Professor, Dhaka College, Dhaka for their participation in the translation process of FAACT ACS Bengali version. They also acknowledge Shamima Rahman, SSN, NICRH, Dhaka, for analyzing the data.
IC: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources and software, validation, writing original draft, editing, and review. YF: Conceptualization, funding acquisition, methodology, supervision, validation and writing original draft. SS: Conceptualization, methodology, supervision, writing original draft, editing, and review. FM: Conceptualization, methodology, supervision, writing original draft, editing, and review. SA: Conceptualization, methodology, supervision, writing original draft, editing, and review. KN: Conceptualization, methodology, supervision, writing original draft, editing, and review.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is funded by Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
Written informed consent was obtained from all subjects before the study.


