Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Daniel Geleta1*, Wubalech Temesgen2 and Netsanet Workneh3
Received: March 29, 2024; Published: April 11, 2024
*Corresponding author: Daniel Geleta, Department of Tropical & Infectious Diseases Jimma University, Jimma, Oromia, Ethiopia
DOI: 10.26717/BJSTR.2024.56.008791
Background: Trachomatous trichiasis, an advanced stage of trachoma infection, can be treated and prevented. It primarily affects economically disadvantaged and marginalized individuals, emphasizing the need for reliable and up-to-date data. However, countries like Ethiopia struggle to consistently gather data on trachoma elimination progress due to the infection's aggressive nature. Thus, the objective of a systematic review and meta-analysis was to present a comprehensive overview of trachomatous trichiasis prevalence in Ethiopia, with the aim of aiding trachoma elimination efforts.
Methodology: A systematic search was conducted in electronic databases to identify peer-reviewed journal papers published in English between January 2015 and December 2020. The search specifically targeted studies that reported the prevalence of trachomatous trichiasis among the adult population in Ethiopia. Three reviewers independently assessed the quality of the included studies, and a standardized charting form was used for data extraction. The synthesized data were subjected to both qualitative and quantitative analysis. The quantitative results were pooled, and an exploration of sources of variation was undertaken. Furthermore, a statistical meta-analysis was conducted using STATA version 16.0 to examine potential sources of influence on prevalence estimates and to assess publication bias.
Results: After excluding 121 duplicate entries, 108 irrelevant titles and abstracts, and removing three articles based on reasons identified during full-text screening, a total of ten eligible cross-sectional studies were included for analysis. The primary outcome of interest in all included studies was the prevalence of Trachomatous trichiasis. The overall pooled prevalence of Trachomatous trichiasis was found to be 1.80% (95% CI = 1.15, 2.44). The individual prevalence estimates from the studies ranged from 0.5% (95% CI = 0.42, 0.60) to 3.9% (95% CI = 3.82, 3.98). On subgroup analysis, the highest prevalence of 3.76% (95% CI = 3.20, 4.41) was reported in Gambella, while the lowest prevalence of 0.5% (95% CI = 0.42, 0.60) was reported in Somalia. The overall trend of the prevalence demonstrated an unstable pattern.
Conclusion: The prevalence of trachoma trichiasis in all regions of Ethiopia exceeds the elimination threshold, highlighting the urgent need for additional implementation efforts.
Keywords: Elimination; Ethiopia; Meta-Analysis; Prevalence; Review; Trachoma Trichiasis
Abbreviations: CI: Confidence Interval; CBCS: Community-Based Cross-Sectional Study; ETP: Estimate Trachoma Prevalence; TF: Follicular Inflammation; TT: Trachomatous Trichiasis; WHO: World Health Organization
Trachoma is a neglected tropical disease that primarily affects impoverished and marginalized populations worldwide. It is caused by an infection with the bacterium Chlamydia trachomatis, which spreads through personal contact and by flies that have come into contact with the discharge of infected individuals within a community. The disease typically manifests as inflammation of the conjunctiva during early childhood, leading to scarring, corneal opacity, and ultimately blindness in individuals aged 15 years and older [1-5]. To facilitate simple and cost-effective diagnosis, the World Health Organization (WHO) has established a grading scale to assess the characteristics of trachoma infection. This scale includes five distinct stages of increasing severity: follicular inflammation (TF), intense trachomatous inflammation, trachomatous scarring, trachomatous trichiasis (TT), and corneal opacity. Trachomatous trichiasis (TT) represents a spectrum of the disease and is characterized by inward-turned eyelashes due to scarring of the upper eyelids. This stage of the disease typically occurs after active trachoma has been controlled and is more commonly diagnosed in adults aged 15 and above, while it is rarely diagnosed in children [5,6].
The World Health Organization (WHO) has been advocating and leading the implementation of the Surgery, Antibiotics, Facial cleanliness, and Environmental improvement (SAFE) strategy to eliminate blinding trachoma in high-burden countries. This strategy is supported by regular surveys and operational research. However, despite the relatively easy prevention and cure options provided by these strategies, trachoma remains the leading infectious cause of blindness worldwide [7,8]. Various studies have reported that 200 million people are still at risk of trachoma, with 1.8 million people experiencing visual loss, 450 thousand people suffering irreversible blindness, and 3.2 million people requiring trichiasis (TT) surgery to prevent blindness and its subsequent burden.
The impact of trachoma on affected individuals and communities can be assessed by measuring the prevalence of Trachomatous Inflammation-Follicular (TF) in children aged 1 to 9 years, TT in individuals aged 15 years and above, and the overall prevalence among the total population. Regular data summarization from prevalence surveys and research helps identify a country's progress towards trachoma elimination. WHO has used this summarized data to announce several countries that are making significant progress in eliminating trachoma. Countries that meet the criteria of having a prevalence of TT of less than 1 case per 1000 individuals aged 15 years and above, and TF of less than 5% in children aged 1 to 9 years, are awarded a certificate of trachoma elimination [9-11]. However, many countries, including those in Africa, continue to struggle with trachoma due to two main factors: suboptimal evidence generation and poorly controlled trachoma determinants such as sanitation, overcrowding, and poverty. These factors hinder the successful implementation of trachoma control measures and impede progress towards elimination [8,11,12].
To address this global issue, Ethiopia has formed a dedicated task force and has been actively executing a range of initiatives to eliminate trachoma. These efforts encompass conducting surveys and operational research. The task force plays a crucial role in regularly consolidating data and monitoring the implementation progress to ensure that the prevalence of trachomatous trichiasis (TT) falls below the elimination threshold. Unfortunately, trachoma continues to be a leading cause of blindness, particularly affecting disadvantaged populations, with a higher prevalence (6.2% of TT) among individuals aged 15 and older throughout the country [3,4,13].
Individual trachoma research, generation of summarized data on prevalence, and detailed observational studies were all planned to contribute to trachoma eradication. However, there was a lack of summarized data, especially regarding the contemporary prevalence pattern of trachomatous trichiasis (TT) in the Ethiopian population aged 15 years and older. Therefore, this systematic review and meta-analysis aimed to determine the prevalence of TT among the adult population in Ethiopia from January 2015 to December 2020. During the prevalence analysis, the current administrative subdivision of Ethiopia was divided into ten ethno-linguistic territorialities: Afar, Amhara, Benshangul-Gumuz, Gambela, Harari, Oromia, Sidama, Somali, South Nation's Nationalities & Peoples Region (SNNPR), and Tigray. Additionally, two chartered cities, Addis Ababa and Dire Dawa, were included to characterize the prevalence [14].
Protocol and Registration
The plan for this review was developed based on the guidelines provided by the Center for Review and Discrimination [15], as well as a meta-analysis report of observational studies following epidemiological guidelines [16]. The selection of studies was conducted in accordance with the recommendations outlined in the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) [17]. The study protocol was established and registered in the PROSPERO-International prospective register of systematic reviews. Following a comprehensive evaluation of existing reviews on the PROSPERO website, the review was assigned the unique identification number CRD42021260802 by PROSPERO, signifying its endorsement and recognition.
Eligibility Criteria
Peer-reviewed journal papers conducted in Ethiopia between 2015 and 2020 were included in this study. The papers had to be published in English-language journals and involve adult participants. The study included original articles with quantitative, qualitative, or mixed study designs that reported data on the prevalence and determinants of trachoma among adults, regardless of the study setting. Papers that did not meet the inclusion criteria, such as those lacking the numerator and denominator needed for estimating the effect size of prevalence, were excluded from the review. Additionally, narratives, commentaries, implementation reports, and essays were excluded. To ensure the review captured the most recent developments in trachoma intervention activities, it focused on the five most recent years following a significant scale-up of trachoma interventions by various stakeholders. This period was chosen to align with the efforts of the WHO Get2020 alliance. The review also had limitations in terms of the geographic study areas included, as the epidemiologic pattern of trachoma infection can vary greatly depending on the intensity of intervention efforts in each locality [17].
Information Sources, Search Strategies and Selection of Sources of Evidence
For this study, original peer-reviewed articles published in English-language journals between January 2015 and December 2020 was collected. Systematic searches were conducted across various electronic bibliographic databases, including PubMed, Google Scholar, Scopus, HINARI, Web of Science, and Worldwide Science. The searches included articles with full text available as of June 2021. The search strategy used for PubMed is outlined in Table 1. The articles identified in the study were collected and stored in a clipboard before being transferred to the Zotero reference management software. A systematic review was conducted on these articles using specific criteria including participants, context, concept, outcome, and study design. In order to be included, the articles had to meet five criteria. First, they needed to focus on the prevalence of trachomatous trichiasis (TT) among adults. Second, they had to examine the determinants of TT prevalence without co-morbidity, excluding studies that investigated trachoma and other infections simultaneously to determine the independent effect of each factor on the outcome. Studies that examined prevalence among non-adult population groups were also not considered. Third, studies conducted in urban, suburban, semi-rural, and rural settings were included, while those claiming to cover all regions of the country were excluded.
Fourth, at least one outcome or finding from the study had to be included. Fifth, only peer-reviewed articles written in English that utilized original cross-sectional and observational research methods were considered, excluding grey literature, narratives, commentaries, reports, essays, and systematic reviews. Three independent reviewers, Daniel Geleta (DG) with epidemiological experience, Wubalech Temsgen with laboratory background and Netsanet Workneh (NW) with medical experience, reviewed the articles. The initial screening involved evaluating titles and abstracts, followed by a thorough review of the full texts. Any discrepancies between the reviewers regarding study selection and data extraction were resolved through discussion and consensus.
Data Charting and Data Items
A data-charting form was collaboratively created by two reviewers to identify the variables to be extracted. Both reviewers individually charted the data, engaged in discussions about the findings, and regularly updated the data-charting form through an iterative process. After finalizing the article selection, relevant information such as the first author(s), publication year, geographical region, study objective, research design, characteristics of the participants (adults), sample size, measurement methods, and recorded outcomes for analysis were entered into a spreadsheet. The details are presented in (Table 2).
Study Quality and Risk of Bias Assessment
The authors of the study used the Newcastle-Ottawa quality assessment tool scale to evaluate the quality of cross-sectional studies. They also consulted an existing tool for assessing the risk of bias in prevalence studies to evaluate inter-rater agreement. The Newcastle-Ottawa tool has three indicators. The first indicator assesses the methodology of the study and is graded out of five stars. The second indicator assesses the comparability of the studies and is graded out of three stars. The last indicator measures the quality of the original articles in terms of their statistical analyses and is graded with two stars. For the analysis, cross-sectional studies with medium (fulfilling 50% of quality assessment criteria) and high quality (≥6 out of 10 scales) scores were included. The first author assessed the quality of the studies and obtained validation from the second author. Additionally, an existing quality assessment tool was used for assessing the quality of observational studies [18].
Data Synthesis and Analysis
A narrative account was created to summarize the included studies and present the combined prevalence and factors associated with TT. The prevalence data was then visualized using a line graph, which showed the relationship between prevalence and publication year. The graph organized the prevalence data chronologically, allowing for the identification of any existing patterns in the infection rates over time. Additionally, the studies were grouped based on the administrative territories (regions) of the respective countries to highlight the heterogeneity of the studies across different regions.
Statistical Meta-Analysis
The researchers conducted a meta-analysis to determine the pooled prevalence of TT using stata-16 software. They employed both fixed-effects and random-effects models to calculate the pooled prevalence. A forest plot was used to visualize the individual and pooled prevalence of the studies, along with their corresponding 95% Confidence Intervals (CI), based on methodological similarities. The statistical heterogeneity of the included studies was assessed using the Q-static test and I2 statistics, along with their corresponding 95% CI. Heterogeneity values falling within the 75-100% quartiles were considered significant. The fixed-effects model was used when the data demonstrated heterogeneity within the two lower quartiles, while the random-effects model was applied otherwise. To examine the trends of TT over the review years, a meta-regression was performed based on the individual study prevalence weights that contributed to the pooled prevalence. Subgroup analysis was conducted using region, sample size, and year of publication to detect heterogeneity. Furthermore, meta-regression was utilized to explore potential sources of heterogeneity. The researchers also conducted sensitivity analyses by excluding each study from the meta-analysis to assess the impact of individual studies on heterogeneity. Additionally, reporting and publication bias were assessed using a funnel plot, and the potential source of publication bias was examined using Egger's tests.
Search Results and Selected Source of Evidence
The electronic database searches yielded a total of 253 records, with 134 from PubMed, 116 from Worldwide Science, and 3 from other sources. Prior to the titles and abstracts screening, 132 duplicate records were removed, resulting in 121 studies being excluded. During the title and abstract screening stage, 108 articles were further eliminated, leaving us with 13 studies for full-text assessment. These 13 full-text articles were obtained from various libraries. Upon conducting the full-text screening, we identified 10 articles that were relevant to our review. No additional articles were excluded after the full-text assessment, as indicated in Figure 1 of the flowchart. Throughout the removal and exclusion process, the main reasons for exclusion were the focus of the study [18-25], the study site [21,26-29], and the target study population [20,22-29]. Consequently, a total of 13 articles were included in our comprehensive data evaluation. However, three articles [30,31-32] were excluded due to their target population and lack of relevance to the review's content. Ultimately, ten articles underwent quality appraisal, data abstraction, and were used for narrative account and meta-analysis stages [13,33-41].
Table 2 provides a summary of the included studies, organized according to the following criteria: first author, year of publication, study region, study purpose, study design, sample size, response rate, study outcome, and the specific outcomes that were analyzed. The included studies consist of a combination of earlier publications and more recent ones. Five articles were published in 2016 [33-37], while three and two studies were published in 2018 [40,41] and 2019 [13,41], respectively. The publications covered various regions, with two studies from the Amhara region [13,39], and one each from Gambela [33], Tigray [34], Beneshangul [35], Somalia [42], Afar [38], SNNPR [43], and Oromia [35]. One study was conducted at a national level, covering a wider area of the country [13]. The majority of the included studies (50.0%) were published in 2016 [32-36], and no publications were identified or included for the years 2015, 2017, and 2020 (Figure 2).
Description of Included Studies
Despite regional variations, all the included studies followed a similar protocol to define the characteristics of their participant population. Each study employed a cross-sectional design without repeated measurements or control groups, with the primary aim of estimating trachoma prevalence. None of the reviewed articles specifically investigated the determinants of trachomatous trichiasis among the adult population during the review period in the respective study areas listed in Table 2. The study purposes of all the included articles were motivated by the need to identify the prevalence of trachoma using a community-based cross-sectional survey (CBCS). The sample sizes varied across the studies, ranging from 3,781 [33] to 241,139 [13], with no reported response rate for any of the included studies. Data collection was conducted at the household level within areas with a total population ranging from 100 to 250,000, serving as the evaluation unit. The prevalence of trachoma and the simultaneous analysis of TT and trachomatous inflammation follicular (TF) prevalence were considered as outcomes in each evaluation unit [13,33-41].
Critical Appraisal Within Sources of Evidence
The included studies underwent a critical appraisal process to assess their quality in terms of participant selection, outcome of interest, and reported findings, as indicated in Table 2. Each study was evaluated for the use of scientifically appropriate sampling methods, adequate sample sizes, and appropriate data analysis techniques. The appraisal was conducted independently by multiple reviewers, using a predetermined format. After completing their individual assessments, the results were compared among the reviewers. Ultimately, all the studies received a strong quality rating of greater than five (>5), as indicated in the right column of (Table 2) [13,33-41].
Pooled Prevalence of TT and Contribution of Region
The findings of a systematic review conducted in Ethiopia indicate that the overall prevalence of trachomatous trichiasis (TT) among the adult population was 1.8% (95% CI = 1.15, 2.44). However, it is important to note that the review identified a high level of significant heterogeneity (I2 = 99.81%, p < 0.001) among the included studies. Each individual study reported varying prevalence estimates, ranging from 0.0% (95% CI = 0.42, 0.60) to 3.9% (95% CI = 3.82, 3.98). These variations were reflected in the forest plot, where the corresponding weight contributions ranged from 9.29% to 10.11% (Figure 2). Significant variations were observed in the contribution of each region to the overall pooled prevalence of trachomatous trichiasis (TT). According to the included studies, Abashawl reported a contribution of 3.76% (95% CI = 3.17, 4.41) from the Gambella region [44], while Tigray had a prevalence of 1.6% (95% CI = 1.45, 1.75) based on the study by Sherif [45], and Beneshangul reported a prevalence of 1.3% (95% CI = 1.15, 1.46) according to the study by Adamu [34]. Other reported prevalence rates included 0.82% (95% CI = 0.77, 0.87) in Oromia [35] by Bero, 1.49% (95% CI = 1.43, 1.56) in SNNPR by Adera [36], 1.4% (95% CI = 0.93, 1.38) in Afar by Nagash [53], 1.7% (95% CI = 1.53, 1.89) in Amhara by Nash [38], 3.9% (95% CI = 3.82, 3.98) in Somali by Duale [55], 0.5% (95% CI = 0.42, 3.98) in Amhara by Stewart [13], and 1.9% (95% CI = 1.84, 1.95) at the national level by Mecleod [40] (Figure 2). Furthermore, the prevalence of TT displayed an unstable pattern of change over the five-year review period. Figure 3 demonstrates a high prevalence in 2016, a decline in 2018, a subsequent increase in 2019, and another decline starting in 2019.
Subgroup Analysis and Investigation of Heterogeneity
To investigate the variation among different regions in Ethiopia, a subgroup analysis was performed based on the administrative division of the country. A random-effects model was utilized for this analysis. Figure 4 illustrates the results of this subgroup analysis. Among the nine regions examined, the Gambella region exhibited the highest prevalence of trachomatous trichiasis (TT) at 3.76% (95% CI = 3.20, 4.41), followed by the Amhara region with a prevalence of 3.50% (95% CI = 3.42, 3.57). On the other hand, the Oromia region had the lowest prevalence at 0.82% (95% CI = 0.77, 0.87). Significant heterogeneity was observed between the regions, as indicated by an I2 value of 99.81% and a p-value of less than 0.001 (Figure 4). It is important to note that since each region was represented by only one or two candidate articles, the findings regarding intragroup heterogeneity remained consistent with the overall pooled prevalence results depicted in Figure 4.
Sensitivity Testing
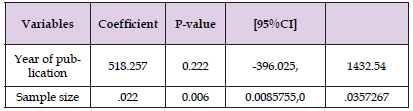
A sensitivity analysis was conducted to evaluate the stability of the pooled effect in the meta-analysis. Each article was systematically excluded, but overall, there were no significant changes observed in the results. However, it is important to note that two studies [13,33] did have a slight impact on reducing the effect size. Further analysis was performed using univariate meta-regression analysis followed by multivariate analysis. Two variables, namely the year of publication and sample size, were examined. The results indicated that the sample size was identified as a potential source of heterogeneity, suggesting that variations in sample sizes across the studies may have influenced the overall effect size. On the other hand, the year of publication did not show a significant association with the observed heterogeneity. Detailed information can be found in Table 3.
Table 3: Meta Regression analysis output on factors associated with heterogeneity of prevalence of TT among adults in Ethiopia.
Publication Bias
Publication bias was evaluated using a funnel plot, which is commonly recommended to assess the presence of bias in meta-analyses. As a general guideline, at least 10 studies are typically required to differentiate between a truly symmetrical distribution and one that occurs by chance [46]. In this analysis, a total of ten articles were included in the meta-analysis. The funnel plot displayed a symmetric distribution of the studies, indicating the absence of evidence for publication bias (Figure 5). This suggests that the included studies were not selectively published based on their results, providing more confidence in the reliability of the meta-analysis findings.
In the present systematic review and meta-analysis, researchers conducted a comprehensive evaluation of published data concerning the prevalence and factors influencing trachomatous trichiasis (TT) in adults aged 15 years and above in Ethiopia. Throughout the review, it was observed that extensive trachoma elimination activities took place in Ethiopia between 2015 and 2020 [13]. However, these activities lacked extensive data on large sample sizes and did not employ robust study designs to examine the determinants of TT. Most of the identified articles were carried out by local trachoma implementing partners with limited involvement of academic institutions, and the prevalence of trachoma, including TT, was primarily assessed using cross-sectional study designs [13,33-41].
The overall pooled prevalence in the country was found to be 1.80%, which exceeded the international elimination threshold of less than 1% [7]. Specifically, the prevalence of trachomatous trichiasis (TT) was found to be higher in certain regions of Ethiopia compared to the overall pooled prevalence. In Gamibella, Amhara, and at the national level, the TT prevalence was approximately 1.96%, 1.7%, and 0.1% higher, respectively, than the pooled prevalence. However, in regions such as Oromia, Benshangul-Gumuz, Afar, Tigray, Somalia, and SNNPR, the TT prevalence was lower than the pooled prevalence. When compared to the World Health Organization (WHO) trachoma elimination threshold of 1% per 1000 population, the pooled prevalence was 0.8% higher [2]. Furthermore, in comparison to other countries, the current pooled result was higher than the study reports in Senegal [48] but lower than the study results conducted in Kenya [47]. These variations can be explained by several factors, with the predominant reasons being the historical endemicity of trachoma in the area and the level of trachoma intervention activities in a particular region [15].
Additionally, the time period of the study could be significant, particularly in Gamibella and Tigray, as the studies were conducted in 2016, while the implementation activities have continued to date, resulting in a significant reduction in prevalence. The prevalence of TT also varied among different regions, and the sample size was identified as a possible source of variation. Although population-based survey design parameters were uniformly endorsed for all trachoma endemic countries, individual countries may modify the design based on factors such as topography, the healthcare system, environmental sanitation, and characteristics of the study population [49]. Despite the regional variations, the results have been utilized to assess the progress of trachoma program implementation in relation to the WHO trachoma elimination threshold and have contributed to elimination activities [13,33-41]. According to the trachoma program guidelines, surveys are expected to be conducted every six months following trachoma-related activities to monitor and control cases [50-64] of TT and contribute to general trachoma elimination efforts.
However, the review revealed that the number of studies conducted in recent years has been even lower than in earlier years, while the prevalence of TT has shown an inconsistent increase. In comparison to other regions, the Amhara region published two of the included articles and was recognized as the region with a higher number of research projects, despite the overall scarcity of research in the country [30,33]. This observation may be attributed to the higher prevalence of trachoma in that particular region [63]. The included articles in the review examined the prevalence of trachomatous trichiasis (TT) across different age groups, using self-reported responses as the primary data collection method. However, this approach is considered ontologically inconvenient as it may introduce biases. None of the studies incorporated laboratory tests to confirm the presence of infection. Additionally, many of the articles did not provide detailed statistical evidence or state tool validation for outcome measurement [13,33-41].
The studies were conducted at the household level, with the district serving as the evaluation unit. The prevalence of TT was estimated at the regional level, which informed decision-making regarding the continuation or discontinuation of trachoma intervention activities at the community level. The sample sizes used in the studies varied across regions, ranging from a minimum of 3,781 participants in Gambella [48] to a maximum of 208,265 participants in the Amhara region [13]. Despite similar objectives and study designs among the included studies, the standard definition of response rate, which is typically acceptable at 10-15% for surveys, was not reported. Furthermore, the analyzed articles focused solely on morbidity as the outcome and did not explore additional analyses that could have provided further insights. However, these reviewed articles have been instrumental in assisting trachoma elimination activity implementing partners in determining the prevalence of TT in the field and implementing appropriate TT elimination measures [16,64].
Strengths and Limitations of the Study
The review was carried out by two reviewers with the aim of summarizing and preparing the data for decision-making during the crucial period of trachoma elimination. The review encompassed all relevant evidence and included only studies of high quality. However, the number of included studies was limited, and some of them lacked important variables, which restricted the scope of certain analyses. Additionally, due to the scarcity of systematic reviews and meta-analyses in the specific area of study, we compared the current findings with the results of non-systematic reviews and meta-analyses studies.
In conclusion, the systematic review and meta-analysis reveal that trachomatous trichiasis (TT) remains a significant public health concern in all regions of Ethiopia, surpassing the WHO elimination threshold. The prevalence of TT varies across regions, with most regions exhibiting higher rates than the overall average and displaying an unstable trend. It is imperative to conduct high-quality research to gain a better understanding of the current patterns of trachoma among adults in Ethiopia. Observational studies are recommended to investigate temporal factors influencing trachoma elimination and guide targeted interventions. Implementers should take immediate action and implement measures to prevent the occurrence and transmission of trachoma. Addressing the determinants of TT and developing focused interventions are crucial steps toward eliminating trachoma as a public health problem in Ethiopia.
Ethical Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
All relevant data are within the paper and its supporting information files.
The authors declare that they have no competing interests.
There was no specified source of fund for the included sources of evidence, as well as the scoping review.
DG has conceptualized and designed the study, while DG and NW equally contributed to the analysis, interpretation, and reporting of the review. Additionally, DG, NW, and WT were jointly involved in literature search, study selection, data extraction, quality assessment, and manuscript write-up. The final manuscript version was approved by DG, NW, and WT, and DG was given the role of correspondence.
There is no special acknowledgment.


