Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Luana Clerico1*, Francesca Cozzi2, Barbara Tonini3, Simona Cannas4, Paolo Emidio Crisi5, Giulia Pignataro5, Benedetta Belà5 and Alessandro Gramenzi5
Received: April 15, 2024; Published: April 25, 2024
*Corresponding author: Luana Clerico, Consultant in Medical Writing and Animal Nutrition, via Castellani, 17043 Carcare – Savona, Italy
DOI: 10.26717/BJSTR.2024.56.008834
Advances in lipidomics revealed the crucial role of fatty acids in physiological and pathological conditions. Imbalances in fatty acid biodistribution might drive the development of canine cognitive dysfunction (CCD), a disabling disease requiring multidisciplinary treatments. There is a growing focus on nutraceuticals, being adjuvant therapies with negligible adverse events. This paper evaluates the effect of Neurogen Pet Ultra® supplementation in aging dogs with CCD. Twenty-four CCD dogs were administered with Neurogen Pet Ultra® for 60 days, followed by 60 days without supplementation. The erythrocyte membrane lipidome was examined in all 24 dogs at the study entry while in the 12 patients who completed the study, even at days 60 and 120. A cluster of 10 fatty acids was quantitatively analyzed using gas chromatography. Diseased dogs’ fatty acid values were statistically compared with those of 8 senior healthy dogs. Dogs with CCD displayed alterations of the fatty acid profile, mainly represented by the increase of saturated and monounsaturated fatty acid families (p = 0.0011 and 0.0194, respectively) and the decrease of the polyunsaturated family (p = 0.0009). Nutraceutical supplementation resulted in a statistically significant normalization of the content of nearly all fatty acids. Lipid modifications in treated dogs were evaluated throughout the follow-up period, showing marked improvements over time. The capability of Neurogen Pet Ultra® to remodel the lipid imbalance confirms its favorable effect on CCD dogs. Moreover, these findings provide new tools for an accurate disease diagnosis, along with the validation of an unbiased method to monitor any therapy.
Keywords: Canine Cognitive Dysfunction; Nutraceuticals; Lipidomics; Erythrocyte Membrane Fatty Acids
Abbreviations: AA: Arachidonic Acid; AD: Alzheimer’s Disease; CADES: Canine Dementia Scale; CCD: Canine Cognitive Dysfunction; DGLA: Dihomo-Gamma-Linolenic Acid; DHA: Docosahexaenoic Acid; EPA: Eicosapentaenoic Acid; FAMEs: Fatty Acid Methyl Esters; GC: Gas Chromatography; LA: Linoleic Acid; MUFA: Monounsaturated Fatty Acids; PC: Phosphatidylcholine; PI: Peroxidation Index; PUFA: Polyunsaturated Fatty Acids; RBC: Red Blood Cells; SFA: Saturated Fatty Acids; SD: Standard Deviation; TLC: Thin Layer Chromatography; TLR: Toll-Like Receptor; UI: Unsaturation Index
The population of aging dogs has grown significantly over the last decades. There are more than 30 million senior and geriatric dogs over the age of seven years in the USA and over 15 million in Europe [1]. Dogs live much longer due to the excellent care level and preventive examinations [2]. However, prolonged life expectancy frequently accompanies age-related diseases such as the Canine Cognitive Dysfunction (CCD) [3,4]. The CCD estimated prevalence ranges from 14% to over 60% and increases as dogs age, even if small breeds are at higher risk because of their greater lifespan [5,6]. Canine cognitive dysfunction is a neurodegenerative syndrome of older dogs characterized by a gradual but progressive cognitive decline [7-9]. Clinical signs are a continuing cognitive impairment and changes in daily routine, including disorientation, altered social interactions, change in sleepwake cycle, aimless wandering, barking, house soiling, alterations in activity level, and anxiety level changes [10,11]. The first approach to diagnose CCD is a clinical evaluation aimed at excluding any systemic or neurologic disease that might mimic CCD symptoms [12]. Diagnostics may require physical and neurologic examinations, bloodwork, thoracic radiographs, and abdominal ultrasound, followed in some cases by a brain MRI and cerebrospinal fluid analysis to evaluate for structural changes such as brain atrophy or inflammation, infection, and neoplasia [13]. The establishment of an accurate diagnosis is often reached with the help of validated screening questionnaires that are related to the owner’s perception, sound tools to rate different stages of CCD and to distinguish this disease from a simple decrease in psychomotor activity caused by general aging of the organism [14- 18].
Canine cognitive dysfunction resembles human Alzheimer’s disease (AD) [19,20], sharing many similarities in the neuropathological abnormalities associated with the disease process [21-24]. Among the pathological brain alterations commonly witnessed in both humans and dogs with cognitive dysfunction, there are cerebrovascular diseases, neuronal mitochondrial dysfunction, oxidative brain damage, impaired neuronal glucose metabolism, astrocyte dysfunction, microglial activation, glutamate-mediated excitotoxic neuronal damage, and abnormal deposition of beta-amyloid plaques [6,8,21,25-27]. Moreover, amyloid beta deposits in dogs have DNA fragments that are highly conserved with those of their human counterpart [28,29]. A-beta amyloid plaques seem to have an essential role in the neuronal death cascade. However, there might be differences between dogs and humans in the persistence of plaques and their distribution, suggesting additional pathogenetic mechanisms in the canine species, such as failure of myelin and synapsis homeostasis and activation of brain damage through immunological mechanisms [24,30,31].
Other common characteristics of CCD and human AD are a loss in synapsis and low plasma and phospholipid precursors levels in the brain [32,33]. In order to hinder cognitive decline and neurodegeneration, the formation of new synapsis, namely synaptogenesis, should be stimulated. This process dramatically relies on the availability of phospholipid precursors to the brain, particularly phosphocholine, an intermediate synthesizing phosphatidylcholine (PC), representing the major component of neuronal brain membranes. In addition, it provides the phosphocholine moiety needed to synthesize sphingomyelin, the other major choline-containing brain phospholipid. Phosphatidylethanolamine synthesis also utilizes ethanolamine instead of choline. At the same time, phosphatidylserine, the third major structural phosphatide, is produced by exchanging a serine molecule for the choline in PC or the ethanolamine in phosphatidylethanolamine [32,34-36]. Phosphatidylcholine synthesis occurs thanks to 3 precursors: docosahexaenoic acid (DHA), uridine, and choline, which reach the brain entirely or predominantly from circulation.
Since the enzymes that synthesize PC act on all of them and have low affinities for these substrates, the blood levels of the three precursors can influence the overall PC synthesis rate [37,38]. The literature demonstrates that brain PC synthesis rapidly increased in rodents fed with a diet supplemented with choline, uridine, and DHA [37,38], while positive outcomes resulted from two clinical trials performed on patients with early AD receiving these three compounds [39,40]. The assumption that the clinical effects of these elements are mediated by an enhanced synaptogenesis is consistent with the observation that the characteristic impairment of connectivity between the varied areas of AD brain was also ameliorated [41]. Therapeutic strategies that may slow the progression and improve signs of CCD include drugs, functional foods, and nutritional supplementation oriented to reduce damage caused by oxidative stress, correct metabolic changes associated with cognitive decline, reduce inflammation, and ameliorate mitochondrial function, neuronal health, and signaling [42].
Strictly adhering to a treatment protocol combining drug treatment, dietary supplementation, and environmental enrichment results in satisfactory clinical outcomes [8]. Dietary supplements, more adequately known as nutraceuticals, have shown a sharp increase in veterinary medicine in recent years [43]. In modern medicine, the administration of nutraceuticals in the prevention and treatment of animal diseases is as joint as it is in humans due to their safety profile, cost-effectiveness, and ease of finding [44]. Nutraceuticals are undefined compounds used to improve the state of health, prevent malignant processes, and control symptoms of different diseases. Their therapeutic components are derived mainly from plants, animals, and marine sources [45] and typically contain the required amounts of vitamins, lipids, proteins, and minerals [46]. Nutraceuticals vary according to their mechanism of action, ranging from an anti-cancer to an antioxidant or anti-inflammatory activity [47]. There is a reasonable body of literature confirming the positive effects of nutraceutical supplementation on neuronal damage and cognition in rat models, dogs, and humans with cognitive impairment [48-55]. However, research needs more objective and unbiased methods to detect the actual effect of nutraceutical treatments.
In 2020, Prasinou et al. disclosed a novel molecular approach helpful in assessing nutritional treatments in health and canine disease conditions [56]. These authors selected a cluster of ten fatty acids representative of cell membranes’ structural and functional properties and quantitatively analyzed them from erythrocyte membrane glycerophospholipids of healthy dogs. These ten fatty acids belonging to saturated (SFA), monounsaturated (MUFA), and polyunsaturated (PUFA) fatty acids families were extracted from red blood cells (RBC) because the RBC composition of fatty acids is informative of fatty acid levels present in various tissues such as muscles, liver, and in tissues not withdrawable during life like retina or brain [57,58]. Lipidomics, the science that studies metabolic pathways through lipids composition [59], has already been widely applied in human research [60-62] but has been scarcely studied in veterinary medicine [63].
Prasinou et al. described the healthy dog lipidomic membrane profile, creating a benchmark for evaluating metabolic and nutritional status in physiological and pathological conditions [56,64]. This study aims to assess whether Neurogen Pet Ultra® dietary supplementation results in significant molecular changes by a quantitative analysis of the lipidomic profile in aging dogs affected by CCD compared to a cohort of senior healthy dogs. Neurogen Pet Ultra® is a nutraceutical product addressed to geriatric dogs and indicated for neuronal protection and neurotransmission regeneration by means of activation of synaptic membranes. It provides the dogs with choline, uridine, DHA, and various antioxidant and anti-inflammatory compounds.
Animals
Twenty-four dogs with signs compatible with CCD were enrolled in this prospective study between January 2019 and January 2020. Dogs with symptoms consistent with cognitive dysfunction were identified through clinical signs and examined by a neurologist (FC). Diagnosis requested the employment of a questionnaire, clinical evaluation, and confirmed in selected cases with advanced diagnostic imaging (brain MRI). The recruited subjects were eligible for the study if they were aged over 108 months in the case of small/medium size dogs or over 84 months in the case of medium/large size dogs. The dogs differed in breed, sex, and body weight (see Tables 1-3). Epileptic dogs and dogs with metabolic or behavioral disorders were equally included in the study, as well as animals already receiving treatments for CCD. Moreover, dog owners must be willing to administer the dietary supplementation accordingly. Exclusion criteria comprised dogs with other concurrent documented diseases that might have signs similar to CCD, such as structural brain lesions (i.e., neoplasia, vascular disease, others); subjects already treated with different nutraceuticals for geriatric diseases; patients in need of further medications due to subsequent medical conditions; dogs presenting adverse events following the dietary supplementation under investigation; animals reporting a deterioration in the clinical status during the observation period. The study protocol did not require any evaluation concerning the type of diet the owners feed their dogs, albeit dietary regimens were registered.
Study Design
Each dog received Neurogen Pet Ultra® (NBF Lanes, Milan, Italy) at the dosage of ½ tablet per 5 kgs live weight once a day for 60 days. Follow-up also included the following 60 days in which Neurogen Pet Ultra® was not administered. Table 4 lists the product components. Animals were not subjected to additional supplementation or change in their daily diet over the observation period. After a first medical examination at study entry (T0), the experimental subjects underwent follow-up visits on day 30 (T30), day 60 (T60), day 90 (T90), and day 120 (T120), when the investigators recorded clinical data. Blood samples were harvested at days 0, 60, and 120 for the lipidomic profile assessment.A control group of 8 healthy elderly dogs, whose fatty acid- based membrane lipidome profile was already studied, has been extrapolated from the Prasinou et al. article [56]. The research was conducted according to the directive 2010/63/EU, article 1 (paragraph 5f); the present study did not imply any form of animal suffering or health risk, since it focused on the administration of a natural substance. However, compliance with the decree ensured a further safeguard for the patient’s health. All dogs enrolled in this study were owned and subjected to regular veterinary checks.
Membrane Lipidome Analysis
Red blood cells (RBC) were isolated from 1 mL of fresh EDTA- treated whole blood sample and used for performing the lipidome analysis as previously described [56,64]. The fatty acid-based membrane lipidome analysis consists of 8 steps, as summarized below and in Figure 1. The process starts with separating erythrocytes from plasma, followed by the washings and centrifugation for membrane isolation that produces a pellet that is suspended in water to extract lipids. The organic layer produced is then separated and evaporated under a vacuum to dryness. A thin layer chromatography (TLC) using chloroform/methanol/water is performed to determine the purity of the phospholipid fraction to avoid plasma contaminants that could interfere with the fatty acid quality and quantity. The phospholipid extract is transesterified at room temperature for 10 min to obtain the fatty acid methyl esters (FAMEs) derived from the fatty acid residues present in membrane glycerophospholipids. The final steps include the extraction of the FAME, their evaporation under vacuum, and the FAME extract’s gas chromatography (GC) analysis. The FAME quantification is performed using CG analysis, as already described [56,64].
The values of the cluster of ten fatty acids representative of the primary fatty acid moieties of the cell membrane are expressed in μg/ mL. Each quantity is reported as a percentage relative to the cluster as the sum of the quantities (100%) that corresponds to > 97% of the peaks found in the GC runs. The fatty acid quantities identified are also helpful for calculating the corresponding lipid indexes by performing mathematical operations of sums and ratios. The cluster of the selected ten fatty acids, including lipid indexes, are detailed in Table 5. The cohort of fatty acids includes palmitic (C16:0) and stearic (C18:0) acids as SFAs; palmitoleic (C16:1), oleic (9c, C18:1), and vaccenic (11c, C18:1) acids as MUFAs; linoleic (LA, C18:2), dihomo-gamma- linolenic (DGLA; C20:3), and arachidonic (AA, C20:4) acids as PUFA omega-6; and eicosapentaenoic (EPA, C20:5) and docosahexaenoic (DHA, C22:6) acids as PUFA omega-3.
These values are also reported as total fatty acid contents (total SFAs, total MUFAs, total PUFAs), and lipid indexes calculated as follows: SFA/MUFA ratio; omega-6/omega-3 ratio; PUFA balance = [(%EPA + %DHA) / Total PUFA] × 100; Unsaturation Index (UI) = (%MUFA × 1) + (%LA × 2) + (%DGLA × 3) + (%ARA × 4) + (%EPA × 5) + (%DHA × 6); Peroxidation Index (PI) = (%MUFA × 0.025) + (%LA × 1) + (%DGLA × 2) + (%ARA × 4) + (%EPA × 6) + (%DHA × 8). As previously published, the GC method can successfully separate all ten fatty acids without overlapping other peaks, particularly the positional and geometrical isomers of the unsaturated fatty acids [56,64].
Statistical Analysis
A statistical analysis was performed using the software GraphPad Prism V.6.01. All data were evaluated using standard descriptive statistics and reported as the mean ± SD or as median and range (minimum- maximum), based on their distribution. Normality was checked using the D’Agostino Pearson test. Variables sequentially collected were compared using repeated measure one-way analysis of variance or a Friedman test according to their distribution, and a post hoc test (Holm-Sidak test or Dunn test) was performed. According to distribution, the data obtained from both groups were compared using an unpaired t-test or Mann-Whitney test. A p-value < 0.05 was considered significant.
Twenty-four elderly dogs were diagnosed with CCD and enrolled in this study between January 2019 and January 2020. In comparison, information on the 8 healthy old dogs staged as a control group originated from a previously published paper [56,64]. Out of 24 sick dogs, only 12 remained in the study until the end of the follow-up (T120), so these last 12 dogs acted as the treatment group to evaluate the effect of Neurogen Pet Ultra®. The distribution of the 32 dogs’ characteristics at baseline is detailed in Table 1, Table 2, and Table 3; the three groups are largely comparable. The dogs belonged to different breeds, including mixed breeds, both male and female, in some cases sterilized, with varying body weights (range 4,2-40), but all aged over 102 months (range 102-216). The first finding of this study, which was crucial for the subsequent evaluation of the nutraceutical treatment, comes from the comparison between the erythrocyte membrane fatty acids of healthy aging dogs and those of aging dogs affected by CCD at study entry (T0). The quantitative GC analysis of the cluster of ten fatty acids representative of the primary fatty acid moieties present in the cell membrane (see Table 5) displayed meaningful changes in CCD subjects. The distribution of modifications to the lipidomic profile arising from the onset of CCD is reported in Table 6.
Table 1: Demographic characteristics of old dogs with canine cognitive dysfunction treated with dietary supplementation (Neurogen Pet Ultra ®) that completed the follow-up period.
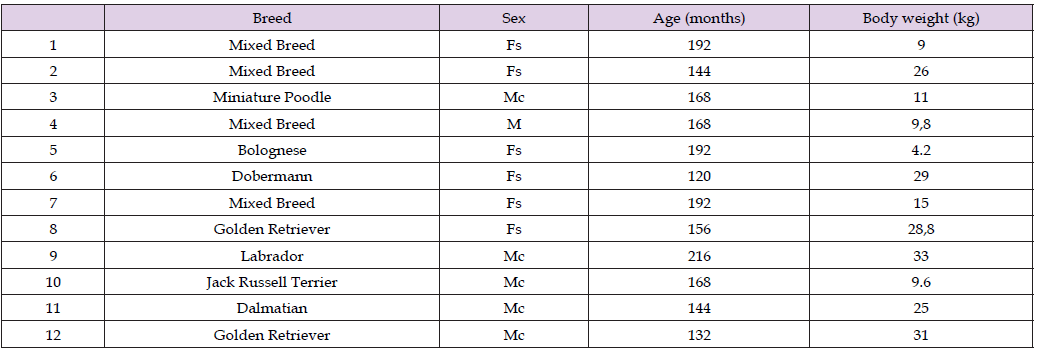
Table 2: Demographic characteristics of old dogs with canine cognitive dysfunction treated with dietary supplementation (Neurogen Pet Ultra®) that did not complete the follow-up period.
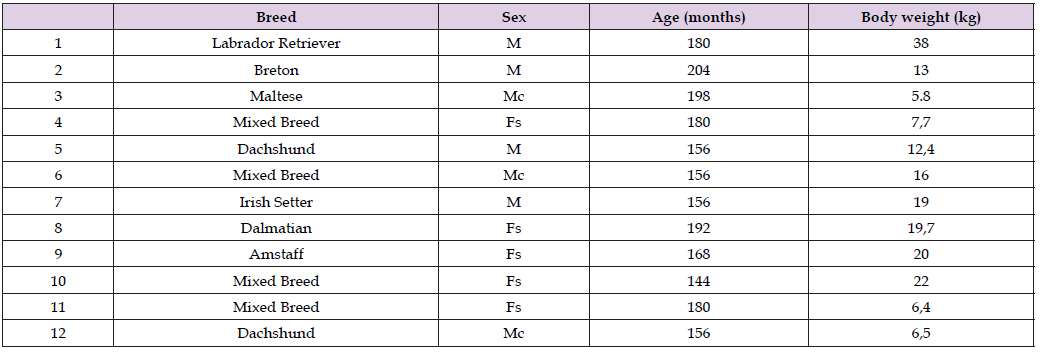
Table 5: List of fatty acids selected for this research, including fatty acid families and lipid indexes.
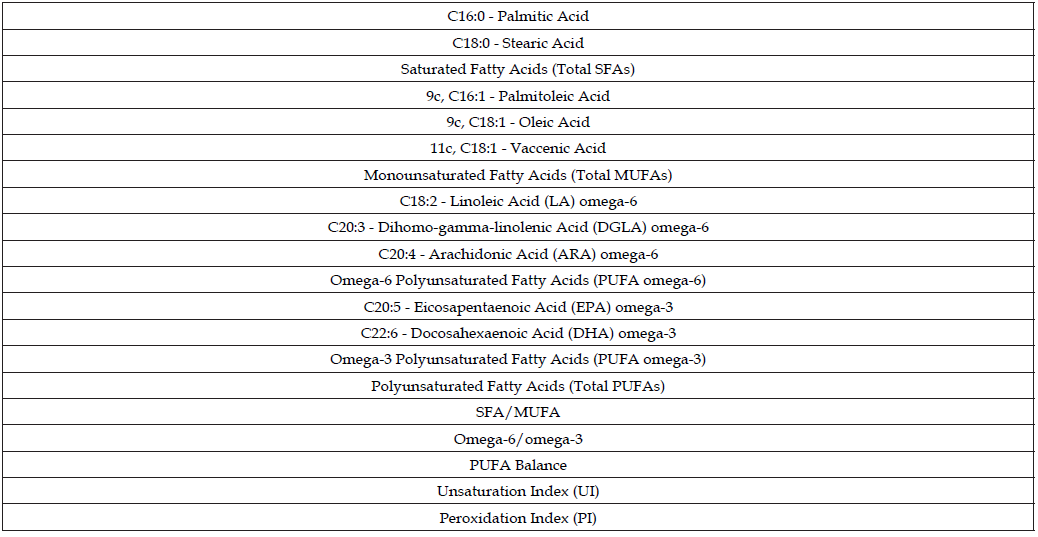
Note: Total saturated fatty acids (SFAs) = % C16:0 + % C18:0.
Total monounsaturated fatty acids (MUFAs) = % 9c, C16:1 + % 9c, C18:1 + % 11c, C18:1.
Polyunsaturated fatty acids (PUFAs) omega-6 = %LA + %DGLA + %ARA.
Polyunsaturated fatty acids (PUFAs) omega-3 = %EPA + %DHA.
Total PUFAs = %LA + %DGLA + %ARA + %EPA + %DHA.
SFA/MUFA = (% C16:0 + % C18:0) / (% C16:1 + % 9c, C18:1 + % 11c, C18:1).
Omega-6/omega-3 ratio = (%LA + %DGLA + %ARA) / (%EPA + %DHA).
PUFA balance = [(%EPA + %DHA) / Total PUFA] × 100.
UI = (%MUFA × 1) + (%LA × 2) + (%DGLA × 3) + (%ARA × 4) + (%EPA × 5) + (%DHA × 6).
PI = (%MUFA × 0.025) + (%LA × 1) + (%DGLA × 2) + (%ARA × 4) + (%EPA × 6) + (%DHA × 8).
Table 6: Mean values of the ten fatty acids derived from the erythrocyte membrane glycerophospholipids of the 8 healthy dogs, and all the 24 dogs affected by canine cognitive dysfunction at Time Zero.
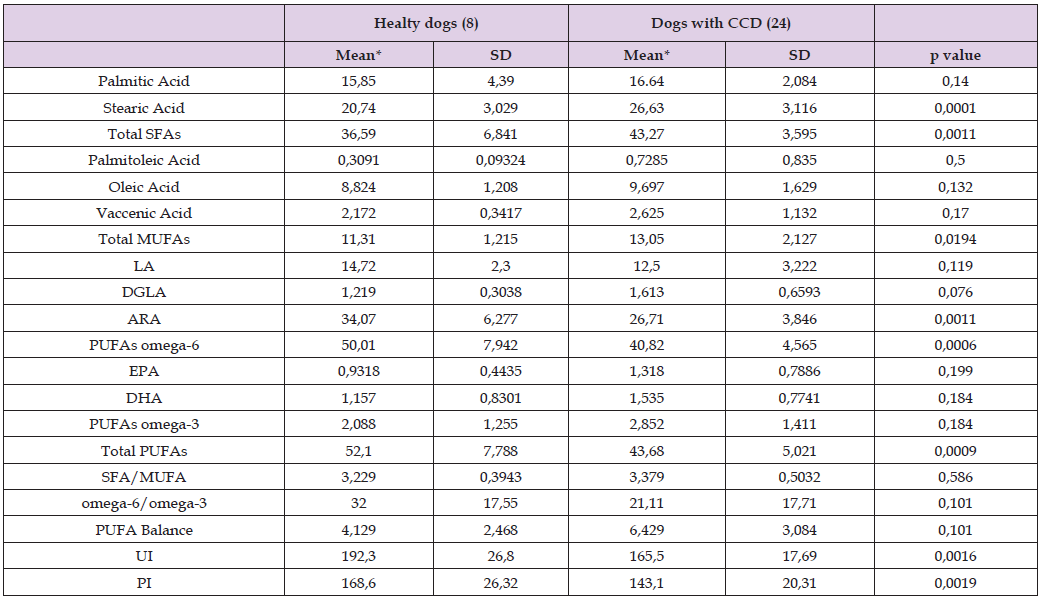
Note: *Values are expressed as μg/mL.
SD = standard deviation.
Specifically, CCD dogs showed a statistically significant increase of stearic acid (p = 0.0001) and total SFAs (p = 0.0011); an increase of total MUFAs (p = 0.0194); a significant decrease of ARA (p = 0.0011), PUFAs omega-6 (p = 0.0006) and total PUFAs (p = 0.0009); a significant decrease of unsaturation (p = 0.0016) and peroxidation (p = 0.0019) index value (UI and PI, respectively). On the other hand, there were no differences between healthy and CCD dogs regarding the levels of palmitic acid, palmitoleic acid, oleic acid, vaccenic acid, LA, DGLA, EPA and DHA, PUFA omega-3, SFA/MUFA ratio, omega-6/ omega-3 ratio, and PUFA balance. Among the 24 CCD dogs, only 12 subjects have gone through the whole follow-up period till day 120 (T120). However, the fatty acid levels at time zero (T0) of this limited cohort of dogs show the same values and the same significant differences compared to healthy dogs (see Table 7), except for DGLA, whose increment reaches the significant level only in the smaller group of dogs that completed the study. Therefore, any DGLA modification after the nutraceutical treatment was not considered valid.
Table 7: Mean values of the ten fatty acids derived from the erythrocyte membrane glycerophospholipids of the 8 healthy dogs, and the 12 dogs with canine cognitive dysfunction that completed the study at Time Zero.

Note: *Values are expressed as μg/mL.
SD = standard deviation.
The second finding that fulfills this study’s main purpose relates to assessing the effect of Neurogen Pet Ultra® in aging dogs affected by CCD. The beneficial effect of the dietary supplementation has been validated using a statistical comparison between the levels of fatty acids under investigation (expressed as μg/mL) in the treatment group at the end of the study period (T 120) and the levels of fatty acids in the control group. Upon completion of the follow-up period (60 days of dietary supplementation plus a further 60 days of observation), the 12 experimental CCD dogs expressed overlapping lipidomic profiles with that of the 8 healthy dogs, as described in Table 8. The unbalance in the fatty acids content following CCD development seems to normalize because of the supplementation with Neurogen Pet Ultra®. Out of 8 parameters that were significantly changed in the treatment group compared to the control group at T0, 7 are within the “normal” range at T120: stearic acid, total SFAs, ARA, PUFAs omega-6, total PUFAs, UI, and PI.
Table 8: Mean values of the ten fatty acids derived from the erythrocyte membrane glycerophospholipids of the 8 healthy dogs and the 12 dogs with canine cognitive dysfunction that completed the study at Time 120.
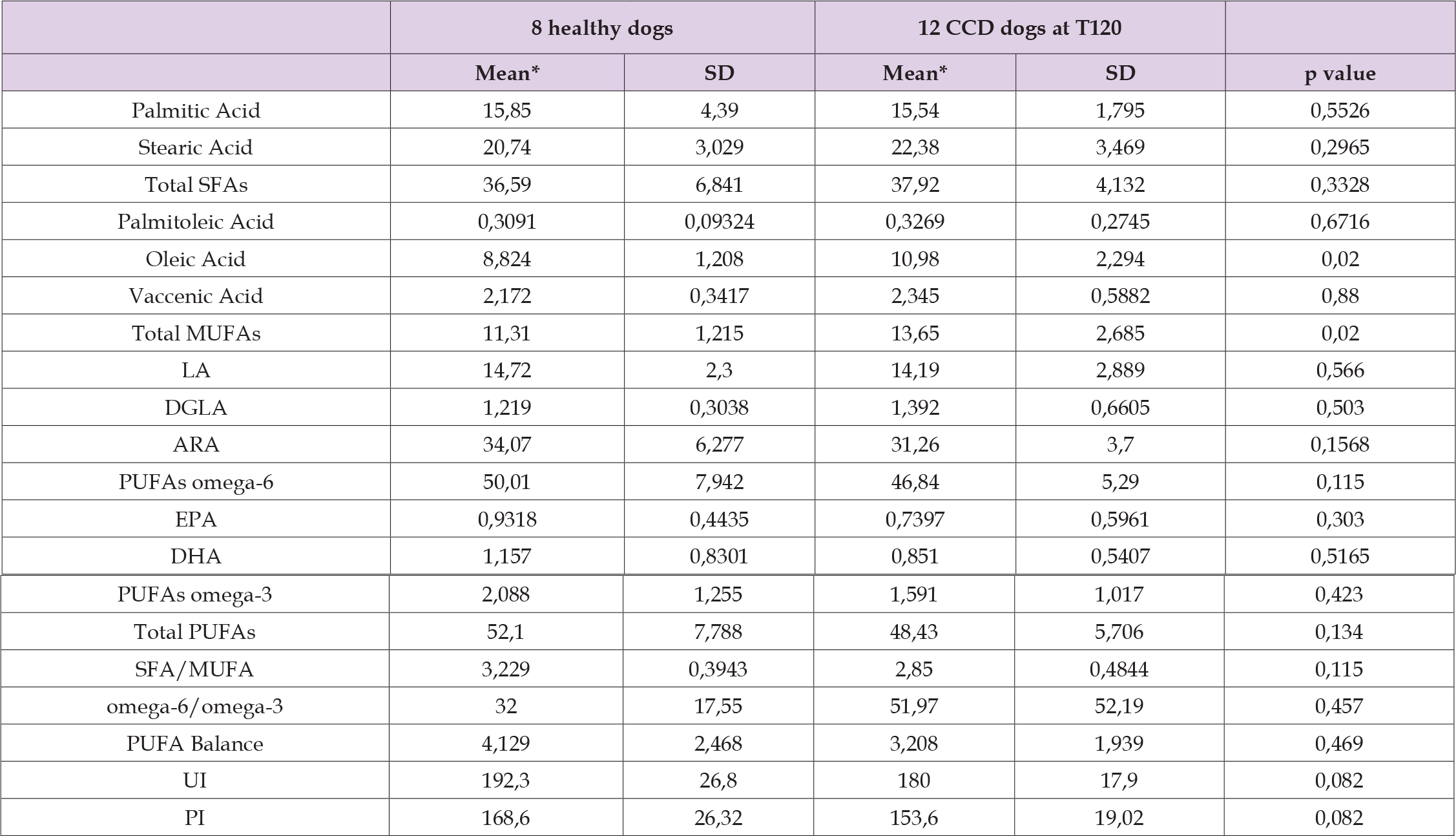
Note: *Values are expressed as μg/mL.
SD= standard deviation.
Total MUFA values represent the unique parameter that did not restore to normal at the end of the study. At the same time, oleic acid increase (p = 0.02) is the only discrepancy induced by the nutraceutical supplementation at 120 days. The final statistical analysis concerns the comparison of amounts of the ten fatty acids (including fatty acid families and lipid indexes) within the treatment group at T0, T60, and T120. Provided that the occurrence of CCD implies a remarkable disparity of the erythrocyte membrane lipidome in aging dogs, noticeable modifications of this imbalance were observed after Neurogen Pet Ultra® supplementation (see Table 9). Indeed, stearic acid decreased significantly at T 120 (p = 0,0087) as well as for total SFAs (p = 0,052), even though this parameter featured a significant decrease already at T60; DHA and PUFA omega-3 values were both declined after the whole follow-up period (p = 0,0038 and p = 0,0278, respectively); SFA/MUFA ratio decreased significantly (p = 0,0023) as well as for PUFA balance values (p = 0,0087) at T120, while omega-6/ omega-3 ratio underwent a substantial increase (p = 0,0087) at the end of the study.
Table 9: Changes in fatty acid values of the treatment group resulting from Neurogen Pet Ultra® dietary supplementation at day 0, 60, and 120.

Note: *Values are expressed as μg/mL.
SD = standard deviation.
Canine cognitive dysfunction is one of the most common neurodegenerative conditions affecting senior dogs [3,23]. With aging dog populations, the prevalence of CCD is increasing [5,65]. However, the pathogenesis of this debilitating illness is still not fully understood, and a multifactorial origin is postulated [9,23,66]. The incidence of CCD does not differ between breeds, nor are there specific differences in clinical signs comparing various breeds [17], so it remains a fact that aging is the most significant risk factor for canine neurodegeneration [4,7]. Although investigators studied many therapies, there are currently no effective pharmaceutical cures for CCD, and the treatment options available are very few, most of them carriers of possible adverse events [67]. As with most canine health and behavior disorders, CCD can be most successfully managed when identified as soon as a dog reaches the clinical threshold for the disease. Therefore, it is imperative to rely on early recognition and intervention [67]. In addition, the treatment of cognitive impairment needs a multimodal approach that includes drugs, balanced diets, supplementations, and environmental and behavioral modification, and it should focus on prevention, maintaining brain health, slowing down cognitive decline, and improving clinical signs [3,67-70]. There is increasing evidence that nutraceutical remedies act similarly to medicines with fewer side effects, which is why nutraceuticals became complementary to drugs, often reducing the dosage of conventional medication or extending its effectiveness [46,71,72]. Not surprisingly, various investigations into nutraceuticals aimed to ameliorate canine cognitive decline have been disclosed in recent years [73-76].
Neurogen Pet Ultra® is a unique nutraceutical product formulated with active ingredients selected on the basis of clinical evidence. It provides geriatric dogs with choline, uridine, and DHA, nutritional precursors needed for synthesizing PC that is essential for membrane phospholipids and for the growth of dendritic processes [32,34-38]. Omega-3 fatty acids supplementation improves membrane physiology and clear improvements in neuronal membrane permeability [76,77]. This product encloses polyphenols and Vitamin E, an antioxidant mix that can hinder the degenerative action of free radicals and counteract oxidative stress by slowing cellular aging [68,78,79]. Neurogen Pet Ultra® also provides animals with curcumin that sharply contrasts neuroinflammation, thus delaying neuronal degeneration [52], and supportive herbal extracts [74].
To evaluate the efficacy of a treatment, investigators routinely rely on validated questionnaires such as CADES (CAnine DEmentia Scale) [14]. These screening tools are a scale rating that monitors the clinical course of the symptomatology during the follow-up period. Limitations to these methods are attributed mainly to pet owners’ perceptions that cannot be unbiased due to their emotional solid involvement; for example, it is common for owners to underestimate their pet’s body condition score and many other clinical or behavioral signs. Several authors have already evaluated using the CADES questionnaire combined with novel biomarkers to predict CCD more readily in elderly dogs [80-82]. Furthermore, recent studies developed and validated a new methodology to support the rapid diagnosis of disease and to objectively assess treatments’ effect in sick dogs using as a benchmark the lipidomic RBC membrane profile of healthy dogs [56,64].
The present study assessed the benefits of Neurogen Pet Ultra® daily administration in elderly dogs with cognitive dysfunction using quantitative analysis of the cluster of fatty acids previously studied by Prasinou and other authors in a subgroup of healthy aging dogs [56]. The first important outcome of this prospective observational study regards the marked changes in the lipidomic profile observed when comparing senior healthy and CCD dogs. In animal models and humans, increased or decreased fatty acid levels are linked to various diseases [64,83,84]. Changes in lipid composition in the case of human AD have already been published [85]. Nevertheless, this cutting-edge evidence is relevant since it comes specifically from research on dogs with cognitive dysfunction. Interestingly, although aging is associated with significant changes in human lipid content [85], the previous analysis on the RBC membrane lipidome of 68 healthy dogs did not show relevant correlations between fatty acid values and age. In fact, the only significant correlation was found with EPA [56]. On the contrary, the development of CCD in this experimental cohort generated significant changes in the abundance of 8 lipid parameters out of 20 analyzed. The overall fatty acid distribution reported in Table 6 points out that the healthy senior dog erythrocyte membrane lipidome is made up almost exclusively of PUFA omega-6 and total SFAs (about 90% of the total RBC fatty acids) followed by total MUFAs, similar to what occurs in other mammalian species [86]. Instead, omega-3 fatty acids account for a minimal concentration, underlining the difference with human lipidome [60,63,86].
Canine cognitive dysfunction promoted a marked and significant increase in SFAs (from 36,59 μg/mL to 43,27 μg/mL; p = 0,0011), mainly driven by stearic acid (p = 0,0001). In humans, SFAs can be regarded as the most harmful type of fatty acids because they can induce the development of atherosclerosis through lipid mediators. High levels of SFAs might be a trigger for insulin resistance and inflammation [87]. Moreover, patients affected by different diseases show a significantly higher proportion of SFAs, being these fatty acids capable of promoting inflammatory signaling by stimulating TLR2 and TLR4 (Toll-like receptors 2 and 4) [88]. Therefore, it can be assumed that an increased concentration of saturated acids in aging dogs is proof of pathological cognitive dysfunction; however, it is also a driving factor for the occurrence of this disease that should be counteracted, possibly with dietary supplementation.
The MUFA pathways, along with SFA and PUFA ones, participate in the specific fatty acid distribution of healthy dog erythrocyte membranes, so it is essential to underline that total MUFAs increased in the RBC membrane of the experimental cohort of CCD dogs (from 11,31 μg/mL to 13,05 μg/mL; p = 0.0194), contributing to the unbalance of fatty acids content. Increased stearic acid levels probably promoted a growth in the monounsaturated acid levels. Polyunsaturated fatty acids represent the other great family of fatty acids fundamental to membrane homeostasis. Omega-3 and omega-6 PUFAs are essential fatty acids for eukaryotic cells, so they are taken from the diet and then enzymatically processed to provide long-chain PUFAs. Feeding dogs with enough omega-6 fatty acids is essential to maintain a good health status. In particular, linoleic acid is necessary to ensure its metabolic transformations to DGLA and arachidonic acid, predominant in dogs, because they are leading structural and functional components of cell membranes [56]. On that basis, it is worthwhile to highlight that CCD dogs here experienced a remarkable reduction in total PUFA content with respect to healthy senior dogs (p = 0,0009), with a highly significant alteration also for omega-6 fatty acids that decreased from a mean value of 50,01 μg/mL to 40,82 μg/mL (p = 0,0006). The evidence that cognitive dysfunction promotes an alteration of the lipidomic profile in aging dogs may relate to the marked decrease of arachidonic acid that drops from 34,07 μg/mL to 26,71 μg/mL (p = 0,0011) even if the mean values of linolenic acid, its precursor, did not significantly change between healthy and CCD dogs. The partial inhibition of this metabolic step that leads to ARA synthesis could indicate a condition of unbalance of the membrane lipids caused by canine cognitive dysfunction.
In this observational study, CCD was even accompanied by a significant decrease in both unsaturation and peroxidation indexes (p = 0,0016; p = 0,0019, respectively). These parameters have already been used for objectively evaluating the homeostasis of membranes in terms of fluidity and oxidative damage [89]. Lipid rafts from the frontal cortex of early AD patients exhibit unusually low unsaturation and peroxidation indexes compared to healthy controls [90]. By setting the two indexes as parameters that mirror an optimal environment for maintaining membrane functions in veterinary medicine, it seems clear that perturbations in the mean values of these indexes indicate a membrane alteration triggered by the disease. Proceeding with the discussion of the results, Table 8 shows the central node of this research, namely the actual clinical effect of Neurogen Pet Ultra® dietary supplementation in CCD aging dogs measured through quantitative analysis of erythrocyte membrane fatty acids. The efficacy of this formulation mirrors the “normalization” of almost all the parameters following the period of treatment with the nutraceutical product. By comparing the fatty acids content between the 8 healthy dogs of the control group and the 12 CCD dogs who achieved the last day of the follow-up period, it was gratifying to see that all SFAs and PUFAs, whether they were checked individually or per family, did not differ in quantitative terms. The unique parameter that remained modified after the treatment was related to the MUFA family (p = 0,02). However, since PUFAs and SFAs are the fatty acid families that mainly contribute to the formation of RBC membranes, it is possible to claim that Neurogen Pet Ultra® is capable of supporting the resetting of the appropriate distribution of fatty acids in the erythrocyte membrane, thus ameliorating its structure and functions. The mean values related to two ratios between the families (SFA/MUFA; omega-6/omega-3) and three indexes (UI; PI; PUFA balance) of the experimental cohort after the treatment period also resulted within the ranges expressed by healthy subjects.
Even the length of the follow-up period is worthy of note: the dogs that completed the study were treated with nutraceutical supplementation for 60 consecutive days; after that, they were observed for an additional 60 days before performing the final molecular-based testing. This protocol was designed precisely for estimating any improvement even after discontinued treatment, thus measuring its benefits over time. Finally, the ability of Neurogen Pet Ultra® to remodel the RBC membrane has been further corroborated by a separate analysis within the treatment group. The fatty acid proportion was evaluated and compared at study entry, after two months, and at the end of the study. Bearing in mind that the starting point was a situation where CCD dogs displayed a fatty acid biodistribution out of balance, the lipidome profile underwent adjustments after 60 days of nutraceutical administration, followed by two additional months of observation. The significant decrease of stearic acid and total SFAs (p = 0,0087 and p = 0,0052, respectively) is desirable given that this fatty acid family is more damaging to the central nervous system. In addition, it should be noted that the reduced amount of SFA family was already significant at day 60.
The last point that requires careful consideration is the change in PUFA omega-3 content. Both DHA and omega-3 family decreased noticeably after dietary supplementation (p = 0,0038 and p = 0,184) despite DHA being integrated. Because of the previous omega-6 reduction triggered by cognitive dysfunction, it can be argued that this omega-3 fatty acid rearrangement is beneficial. Since dogs’ omega-6/ omega-3 ratio is incredibly high and crucial for maintaining specific metabolic effects, this ratio can be restored even through omega-3 lowering. It certainly needs to be emphasized that the SFA/MUFA ratio and PUFA balance went to substantial changes (p values = 0,0023; 0,0087); therefore, these modified lipid indexes stand for an active remodeling process of the RBC membrane structure induced by Neurogen Pet Ultra® intake. We are conscious that this study has some limitations since it has been conducted on a restricted number of patients, while information on the control group was gathered previously. Despite the modern molecular-based methodology proposed, we also acknowledge that a traditional clinical evaluation of the nutraceutical effects is needed to reinforce the results.
The outcomes herein reported fully meet the primary endpoint by providing unbiased evidence of Neurogen Pet Ultra® benefits in the case of CCD. Nevertheless, the implications the study offers in terms of new scientific knowledge are much broader, as we were able to deliver more profound insights into the mechanism of CCD occurrence. Our findings validate the use of membrane lipidome analysis for an early, fast, and accurate diagnosis of canine cognitive dysfunction, in addition to a reliable method for monitoring different therapies. We advocate setting up further studies with this scientific approach to thoroughly investigate any added advantage of dietary supplementation with Neurogen Pet Ultra® in preventing and treating canine neurodegeneration.
The present study objectively discloses the effect of Neurogen Pet Ultra® dietary supplementation in aging dogs affected by canine cognitive dysfunction. One of the strengths of this research is the molecular- based approach performed to demonstrate the nutraceutical effect. Using a tested protocol, the authors examined and quantified a cluster of ten fatty acids in the erythrocyte membrane of a cohort of diseased dogs, being erythrocyte fatty acids composition informative of the brain tissue. The outcomes displayed that Neurogen Pet Ultra® supplementation can significantly adjust the fatty acid alterations caused by cognitive dysfunction in senior dogs, thus supporting the remodeling of the cell membrane structure. Resetting the levels of most lipid components is fundamental to maintaining the functional properties of cell membranes, essential for canine health.
The authors thank Giuseppe Pappini and Carlo Maria Colombo for NBF Lanes financial support.
Two co-authors of the present study, Prof. Alessandro Gramenzi and Dr. Giulia Pignataro, are NBF consultants.
This research was funded by the NBF Lanes Srl, Corso di Porta Vittoria 14, 20122 Milano.


