Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Sushna Maharjan*1, Sabin Ranabhat1, Mamata Tiwari1, Anita Bhandari1, Bidur Prasad Osti1 and Puja Neopane2.
Received: May 05, 2017 Published: May 25, 2017
Corresponding author: Sushna Maharjan, Department of Pathology, Chitwan Medical College Teaching Hospital (CMC-TH), P.O. Box 42, Bharatpur-10, Chitwan, Nepal
DOI: 10.26717/BJSTR.2017.01.000108
Introduction: Sydney System Classification was designed to provide simple practical guidelines for reporting of the endoscopic appearances of gastric mucosa. The “Up-Dated Sydney System” provided a helpful “Visual Analogue Scale” for grading of histological parameters. Data on Helicobacter pylori gastritis is sparse from Nepal; hence this study is conducted to apply the Up-Dated Sydney Classification and grading of H. pylori gastritis for their diagnostic evaluation.
Materials and Methods: Gastric biopsies from 110 patients from February (2015) to July (2016) were analyzed for H. pylori, and Visual Analogue Scale presented by Up-Dated Sydney Classification system was applied. SPSS 16.0 was used for statistical analysis.
Results: Chronic gastritis was observed among age group of 20-82 years in 57 (52%) females and 53 (48%) males. The most common age group affected was 61-70 years. Abdominal pain was the frequent symptom. Antrum was the common site. Fifty nine (53.6%) cases had H. pylori colonization. Mild, moderate and marked density of H. pylori was demonstrated in 41(37.3%), 8(7.3%) and 10(9%) cases, respectively. Mononuclear cell infiltrate was detected in all the cases with H. pylori detection in 59 (53.6%) cases. Neutrophilic activity was observed in 37(33.6%) cases. Twenty four (40.7%) H. pylori positive cases had neutrophilc activity. Intestinal metaplasia and atrophy were observed in 19% and 5.5% of cases, respectively.
Conclusion: The significant correlation was found between the presence as well as concentration of H. pylori colonization with neutrophilic and mononuclear cell infiltrate activity.
Keywords: Atrophy; Gastritis; Helicobacter pylori; Intestinal Metaplasia
Abbreviations: CMC-TH: Chitwan Medical College Teaching Hospital; CMC-IRC: Chitwan Medical College Institutional Review Committee; H&E: Hematoxylin and eosin; H. pylori: Helicobacter pylori; IM: intestinal metaplasia; MNC: mononuclear cells; PAS: Periodic Acid Schiff; p value: probability value; SPSS: Statistical Package for Social Science
A working party presented the Sydney System: A New Classification of Gastritis in Sydney at the World Congress of Gastroenterology in 1990 [1-6]. The discovery of Helicobater pylori by Robin Warren and Barry Marshall (1982) was the revolution in understanding of gastritis and upper gastrointestinal disease [7]. H. pylori infection became evident as the key factor and initiator of the majority of the pathology related to gastritis and its sequelae. Since then interest in gastritis and the morphological appearances of endoscopic biopsies increased. However, H. pylori may not be the sole etiological agent in some cases where H. pylori are detected in biopsies [8,9]. Sydney System Working Party was designed to provide simple practical guidelines for the documentation of histological features of gastritis and also to provide guidelines for the reporting and classification of the endoscopic appearances of the gastric mucosa [3]. The core of the classification was the topography of the gastritis which was restricted to the antrum, the corpus, or a pangastritis. A prefix was to be added if the etiology of gastritis was known (e.g. H. pyloriantral gastritis; autoimmune corpus gastritis etc). Later on Dixon et al. published the “Up-Dated Sydney System” (1997) [10].
This up-dated system added the recommendation to include biopsies from the angulus of the stomach because the presence of intestinal metaplasia (IM) in the angulus may be an early sign of atrophic multifocal gastritis. It also provided a helpful “Visual Analogue Scale” for the grading of the histological parameters. Five key graded morphological variables were to be included as suffix which were chronic inflammation (chronic gastritis), the activity of the gastritis measured by the presence of polymorphonuclear leucocytes, IM, atrophy manifest by the loss of the normal mucosal glands and the presence of H. pylori organisms in accordance to Up-Dated Sydney System proposed in that system which is mentioned in the original publication [11]. These parameters were semi-quantitatively graded as absent, mild, moderate or severe, each successive grade to represent an increase in severity of approximately one third, as per guidelines recommendation. Both the original and the Up- Dated Sydney Systems are still acceptable worldwide. The aim of this study was to evaluate the gastric biopsy material in our institution to:
present the frequency of H. pylori gastritis
Investigate whether or not H. pylori influences the histological severity of chronic gastritis.
The present study is a prospective, cross sectional and descriptive study that included 110 endoscopic gastric biopsies for gastritis received from February 2015 to July 2016 in the Department of Pathology; Chitwan Medical College & Teaching Hospital (Nepal) and analyzed with light microscopy. The protocol had the approval of the Chitwan Medical College Institutional Review Committee (CMC-IRC) and confidentiality of all participating patients was considered for ethical issues. We included the patients of all ages and both sexes having upper gastrointestinal symptoms with histological evidence of gastritis, and excluded the cases with superficial gastric biopsy specimens and those cases which were diagnosed as malignancy. Age and sex of the patients, detailed clinical history and upper gastrointestinal endoscopy findings were obtained from the departmental records regularly.
Endoscopic gastric biopsies- two from antral region were taken from greater and lesser curvature, and two more biopsies were obtained from the additional sites of abnormalities like fundus, body, cardia, and so on by the surgeons and sent for histopathological examination. All four specimens were received in separate containers and all of them were processed separately. Biopsies were fixed in 10% formalin, routinely processed and embedded in paraffin wax. Tissue sections at 5μm thick were cut, stained with hematoxylin and eosin (H&E), and analyzed to assess the histopathological features of gastritis. Giemsa stain was performed to demonstrate [H. pylori for each of the biopsy specimen. The demonstration of intestinal metaplasia was enhanced by using a special stain, Periodic Acid Schiff (PAS) stain. The grades of the major morphological variables H. pylori density, neutrophilic activity, mononuclear cells (MNC) infiltration, IM, and glandular atrophy] were determined using the semi-quantitative method of scoring according to the Up-Dated Sydney Classification system with a Visual Analogue Scale, and scored 0-3 (absent, mild, moderate or marked) to each morphological variable. Minor histopathological features were not graded, but simply assessed if present.
Microsoft Excel database was used to record all data and the results were analyzed by using SPSS 16.0 for Windows. Chi-squared test was used to determine the significance between the variables. A probability value (p-value) < 0.05 was considered statistically significant.
We analyzed 110 cases of gastric endoscopic biopsies. The most common age group affected by chronic gastritis was 61- 70 years constituting 22% of cases, followed by 19% cases each in 31-40, 41-50, and 51-60 years (Table 1). Chronic gastritis was widely distributed among age group ranging from 20-82 years with a mean age of 51 years. There were 57 (52%) female and 53 (48%) male patients with only a slight predominance of female patients showing a female: male ratio of 1.07:1. Patients had upper gastrointestinal symptoms. The most common symptom was abdominal pain, presented by 101 (92%) cases. The patients presented either as abdominal pain only or with other associated symptoms. Other symptoms were dyspepsia (42.3%), upper GI bleeding (3%), vomiting (3%) and belching (2%).
Table 1 : Age of the patients and their H. pylori status in chronic gastritis.

Endoscopic findings were available in 96 (87%) cases. Antrum was the most common site, comprising 62 (64.5%) cases among which 41 (43%) cases were affected in antrum only and remaining had associated lesions in duodenum/ gastroesophageal junction/ fundus/ body/pylorus. Six (6.3%) lesions were found in fundus, 5 (5.2%) cases had pangastritis; 4 (4.2%) lesions were detected each in duodenum, cardia, lesser curvature and gastroesophageal junction; 2 (2.1%) lesions in body, and 1 (1%) lesion in pylorus. The other lesions found in other than antrum affecting two or more sites were- 1 (1%) each case in duodenum and pylorus; cardia, fundus and body; lesser curvature and body; and lesser curvature and cardia. The most common lesion recorded was ulcer in 58 (60.5%) cases. Other lesions were polyps in 22 (23%) cases, erosion in 12 (12.5%) cases, hyperemia in 2 (2%) cases, and diverticula with fistula in duodenum in 2 (2%) cases. Giemsa stained slides demonstrated mild density of H. pylori colonization (Figure 1) in 41 (37.3%) biopsies, moderate density in 8 (7.3%) and marked density (Figures 2 & 3) in 10 (9%) among 59 (53.6%) H. pylori positive cases (Tables 2 & 3) that included 31 females and 28 males. H. pylori was not found in 51 (46.4%) cases (Table 2).

Figure 1: Gastric mucosal gland showing a mild density of H. pylori (arrow) (Giemsa stain x1000).
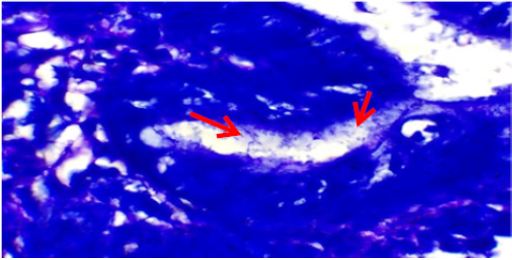
Figure 2: Gastric mucosal gland showing heavy colonization of H. pylori (arrows) (Giemsa stain x1000).
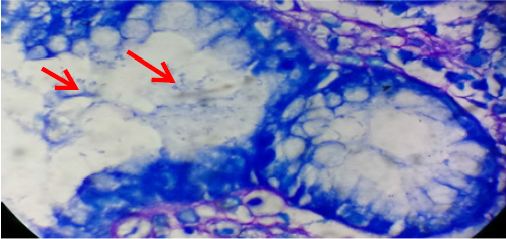
Figure 3: Gastric mucosal gland showing heavy colonization of H. pylori (arrows) (Giemsa stain x1000).
Table 2: Correlation between H. pylori status with the major morphological variables.
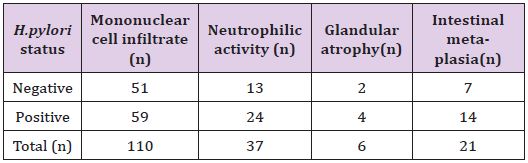
*The result is statistically significant at p<0.05 for MNC infiltrate and neutrophilic activity but not significant for glandular atrophy and IMat p <0.05.
H. pylori was observed in surface alone, both surface and crypts, and crypts only in 36 (61%), 20 (34%) and 3 (5%), respectively. Each fifteen (25.4%) cases of H. pylori positive cases were in age group 31-40 years and 41-50 years followed by 13 (22%) cases each in 51-60 years and 61-70 years, 2 (3.4%) cases in 81-90 years and 1 (1.7%) case in 71-80 years (Table 1). Mononuclear cell infiltrate was detected in all the cases. There was mild, moderate and marked infiltrate (Figure 4) in 15 (13.6%), 64(58.2%) and 31(28.2%) cases, respectively. Two (6.5%) cases out of 31 marked MNC infiltrate had formation of lymphoid follicles with germinal centre. H. pylori was demonstrated in 1(6.7%) case with mild, 32 (50%) cases of moderate and 26 (83.8%) cases of marked MNC infiltrate (Table 3). Only one case with lymphoid follicles had shown H. pylori colonization. The MNC infiltration was associated with plasma cells in most of the cases, i.e. 89 (81%) cases and eosinophils in only 21 (19%) cases. Neutrophilic activity (Figure 5) was observed in 37 (33.6%) biopsies.
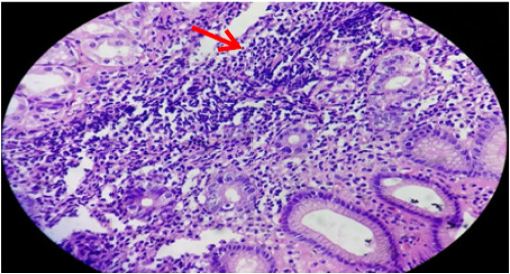
Figure 4: Gastric mucosa showing marked mononuclear cell infiltrates (arrow) in lamina propria (arrow) H&E stain x400).
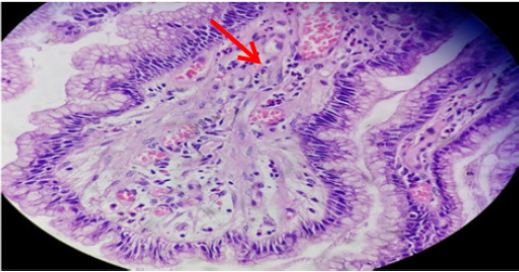
Figure 5: Gastric mucosa showing neutrophilic activity (thick arrow) admixed with mononuclear cell infiltrates in lamina propria (H&E stain x400).
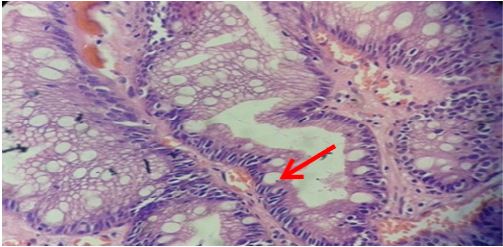
Figure 6:Gastric glands showing marked intestinal mateplasia (arrow) (H&E stain x400).
Table 3: *Correlation of concentration of H. pylori colonization with grade of MNC activity.
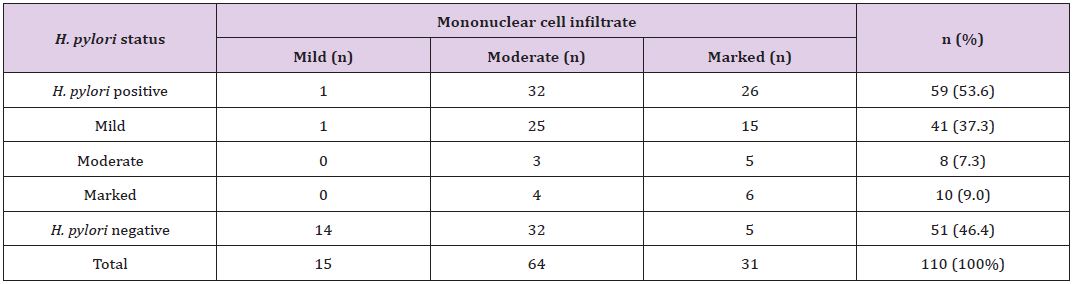
*The p-value is 0.00, statistically significant at p <0.05.
Table 4: *Correlation of concentration of H. pylori colonization with grade of neutrophilic activity.
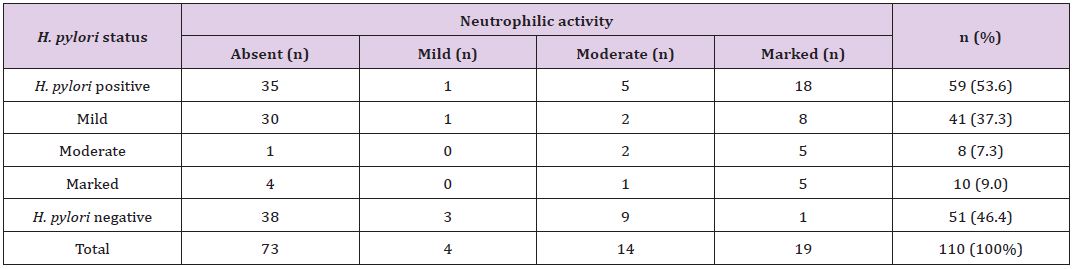
*The p-value is 0.00, statistically significant at p <0.05.
Table 5: *Correlation of concentration of H. pylori colonization with grade of intestinal metaplasia.
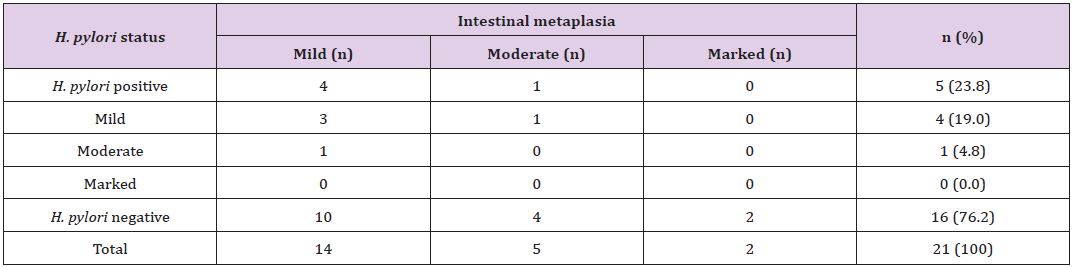
*The p-value is 0.89, statistically not significant at p <0 .05.
Table 6: *Correlation of concentration of H. pylori colonization with grade of glandular atrophy.
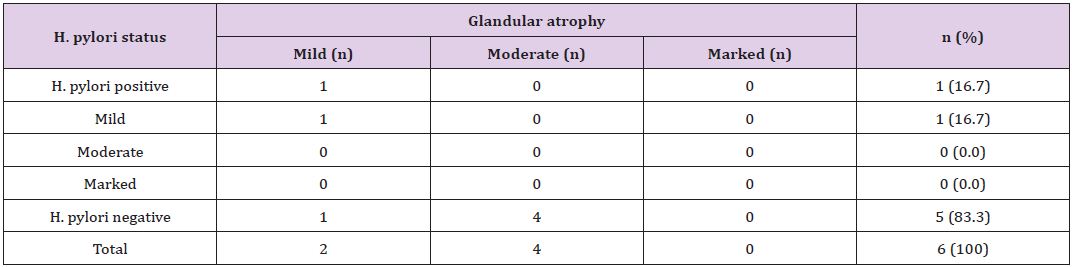
*The p-value is 0.12, statistically not significant at p <0.05.
The degree of neutrophilic activity was mild, moderate and marked in 4 (3.6%), 14 (13%) and 19 (17%) cases, respectively. Neutrophilic activity was present in 24 (40.7%) of H.pylori positive cases. H. pylori was not demonstrated in 13 (35.1%) of cases in which neutrophilic activity was present. H. pylori was positive in 1 (25%) case with mild neutrophilic activity and 5 (35.7%) cases with moderate neutrophilic activity. Expect one case, 18 (94.7%) out of 19 cases with marked neutrophilic activity demonstrated H. pylori colonization (Table 4). Grading of intestinal metaplasia was mild in 14 (66.7%) cases, moderate in 5 (23.8%) cases and marked in 2 (9.5%) cases among 21 (19%) cases with IM (Figure 6). The demonstration of IM was enhanced by using a special stain, Periodic Acid Schiff (PAS) stain. H. pylori was found in 5 (23.8%) cases of IM, i.e, among 4 (28.6%) cases of mild IM and 1 (20%) case of moderate IM (Table 5). Glandular atrophy was detected in 6 (5.5%) cases only. Mild atrophy was seen in 2 (33.3%) cases and moderate atrophy in 4 (66.7%) cases. The colonization of H. pylori was found 16.7% of cases of atrophy, i.e, among 1 case of mild atrophy and none of the cases of moderate atrophy (Table 6). The different histological parameters coexisted in the same patient but in different regions of the stomach. Mononuclear cell infiltrate was observed almost in all specimens and the average of different areas was taken to score the density.
Neutrophilic activity was found in antrum, 31 (83.78%); body, 3 (8.2%); cardia, 2 (5.4%) and fundus, 1(2.8%). IM was detected in antrum, 16 (76.2); body, 4 (19.0) and fundus, 1 (4.76%). Atrophy was found in antrum, 4 (66.7%) and body, 2 (33.3%).
Chi-squared test showed the significant correlation of presence of H. pylori colonization with grades of variables; MNC activity (p<0.00001) and neutrophilic activity (p=0.00) (Table 2). However, there was no significant correlation of presence of H. pylori colonization with grades of IM (p=0.65) and glandular atrophy (p=0.12) (Table 2). There was also statistical significance of quantitative grading (i.e, concentration) of H. pylori colonization with grades of MNC activity (p=0.00) (Table 3) and neutrophilic activity (p=0.00) (Table 4), and no association with grades of IM (p=0.89) (Table 5) and glandular atrophy (p=0.12) (Table 6). Thus, it suggests that density of H. pylori colonization increases with the severity of neutrophilic activity, and mononuclear cell activity. We observed ulcer on histological study in 32 (29%) cases. Other non graded histopathological variables, such as foveolar hyperplasia, regenerative atypia and dysplasia of epithelium were also observed. Foveolar hyperplasia was found in 18 (16.7%) cases in which H. pylori colonization was demonstrated in 11 (61%) cases. Regenerative atypia and dysplasia were observed in 17 cases each.
The most common age group affected by chronic gastritis in the present study was 61-70 years with a mean age of 65.8 years, which is consistent with study performed by Pruthi et al. [12]. However, Garg et al. [13], Aydin et al. [14] and Mustapha et al. [15] had reported chronic gastritis in relatively younger age group with a mean age of 47 years. Udoh et al. [16] also observed chronic gastritis in younger age group with mean age of 48.6 years. We detected only two cases of chronic gastritis below or at the age of 20 years and both the cases were negative for H. pylori colonization. However, the study of chronic gastritis among children in Colombia showed H. pylori infection being common, affecting 59% of total children [17].
The rate of gastric colonization of H. pylori increased with increasing age in the present study, similar to the findings of other studies [18,19]. In contrast, there is a report with increased rate of H. pylori infection in Colombian children [17]. We observed a slight higher incidence rate of chronic gastritis in females with a M:F ratio of 1.07:1. Similarly, chronic gastritis was common in female children as well [17]. In contrast, the other study from Nepal [20] observed a higher incidence in males with a M:F ratio of 1.6:1.Chen et al. [21], Garg et al. [13], and Pruthi et al. [12],and Park et al. [22] also had a higher incidence rate in males with a M:F ratio of 1.8:1, 2.1:1, 2.3:1 and 2.8:1 respectively. H. pylori colonization was detected more in male patients [12-16]. In contrast, prevalence of H. pylori infection was higher in female patients in the present study.
Abdominal pain (92%) followed by dyspepsia (42.3%) were the common symptoms in the present study. Pruthi et al. [12] reported dyspepsia as the common symptom (33.3%) followed by abdominal pain (22.2%). Non-ulcer dyspepsia (84%) was the common clinical condition in the study by Dhakhwa et al [20]. Antrum (64.5%) was the most common site in our study. Similarly, common biopsy location was from antrum in other studies [12-16]. In the study by Dhakhwa et al. [20], Garg et al. [13] and Park et al. [22] tissue was obtained from antrum only. H. pylori colonization was more in the antrum than in the corpus [23]. Endoscopic findings showed antrum ulcers (60.5%) as the common lesion in the present study. Similarly, Pruthi et al. [12] also observed antral ulcers/erosions (46.7%) as common lesion. Garg et al. [13] had reported antrum hyperemia (68%) as the most frequent lesion and duodenal ulcer (60%) was common in the study by Park et al. [22].Our study demonstrated H. pylori in 53.6% of cases of chronic gastritis. Dhakhwa et al. [20] and Pruthi et al. [12] had detected 44% and 47% of cases of chronic gastritis with H. pylori colonization respectively, which is lower than in our study. Archila et al. [17] and Park et al. [22] had found more H. pylori positive cases constituting 59% and 65.5% respectively. The incidence rate of H. pylori in different studies is subject to the accuracy of biopsy techniques. Thus, multiple biopsies are to be taken to improve the yield. The lower incidence of H. pylori in our study could be due to the use of proton pump inhibitors or antimicrobial agents by the patients prior to endoscopic biopsies or not following the proper guidelines to obtain mucosal biopsies. Mononuclear cell infiltrate was observed in all cases in the present study. Similarly, other studies also had MNC infiltrate in all cases [13,17,22,24].
The majority (58.2%) of the biopsies showed moderate inflammation; 28.2% had marked inflammation and 13.6% had mild inflammation in the present study. Likewise, Archila et al. [17], Park et al. [22], and Whitteman et al. [24] observed the majority of cases of chronic gastritis and moderate inflammation. However, Garg et al. [13] found the majority (70%) of cases with mild inflammation. The density of H. pylori colonization increased with severity of chronic inflammation in our study. This finding was compatible to other studies [13,17,25]. In contrast, there was no statistical significant relationship between the density of H. pylori infection and the grade of chronic inflammation in the study conducted by Udoh et al. [16].The present study demonstrated H. pylori in 1 (50%) case out of 2 cases with lymphoid follicles. This finding is consistent with the study by Pruthi et al. [12] and Genta et al. [26] showing H. pylori colonization in 55.2% cases with lymphoid follicles.
In contrast, Dhakwa et al. [20] had 6 cases with lymphoid follicles and H. pylori was positive in all cases. Garg et al. [13] observed lymphoid aggregates in 25% and lymphoid follicles in 19% of cases where the presence of lymphoid follicles was strongly associated with H. pylori infection.
In the present study neutrophilic activity was present in 33.6% of cases. Dhakwa et al. [20] and Park et al. [22] had higher incidence of neutrophilic activity than in our study comprising 50% and 78.7% of cases, respectively. Neutrophilic activity was present in all H. pylori positive cases [22] and 80% of H. pylori positive case [22] but it was observed only in 40.7% of H. pylori positive cases in our study. H. pylori was not demonstrated in 13.4% and 76.7% of cases, respectively [20-22] in which neutrophilic activity was present. In this study, H. pylori were not demonstrated in 35.1% of cases in which neutrophilic activity was present. There was a significant association between neutrophilic activity with H. pylori concentration in the present study as discussed elsewhere [11,13,20,27]. However, Park et al. [22] observed no statistical significance between neutrophilic activity and H. pylori concentration.
Atrophy was present in 5.5% of chronic gastritis and most of the cases were in 5th to 7th decades of life in the present study. Similarly, other studies had shown increase of atrophy with increasing age [12,28].
The other study from Nepal [20] and Garg et al. [13] observed atrophy in 10% and 12.33% of cases respectively which is higher than in the present study. In contrast, other studies had reported rare incidence of atrophic gastritis [29,30]. Atrophy was rarer in children with 0.7% of cases only [17]. However, higher prevalence of atrophy was observed in the other study of gastritis in children constituting 16% of cases [31].In the present study, H. pylori colonization was observed in 16.7% of cases of gastric atrophy which is similar to the study conducted in children [17] but the other study in children identified H. pylori colonization in 100% of cases of gastric atrophy [31]. Pruthi et al. [12] observed H. pylori colonization in 62.5% of cases of gastric atrophy. We found intestinal metaplasia in 19% of cases. Others had reported higher incidence of IM with 34.2% of cases and 36% of cases [12,32]. Garg et al. [13] and Dhakhwa et al. [20] found fewer incidences of IM comprising 7 % and 5% cases, respectively. In children, IM was demonstrated in even fewer cases constituting 1% only [17]. H. pylori was demonstrated in 23.8% cases of IM in the present study. Pruthi et al. [12] had identified H. pylori in 32.2% cases of IM. Intestinal metaplasia and atrophy associated with H. pylori colonization were common in antrum in the present study. Similar finding was observed by Goldstein et al. [33] and Satoh et al [34].There was no significant association of H pylori with IM and atrophy as discussed elsewhere[13,20,24].This finding is compatible with our study.
In the present study the topographic sites according to the Sydney system for H.pylori, neutrophilic infiltration, MNC infiltrate, gastric atrophy or IM was not possible because the Sydney system guidelines was not followed to obtain the mucosal biopsies. The findings could be improved in this study especially, the histological parameters of gastric atrophy and IM if biopsies were obtained from incisura angularis where maximal of these changes occur, and H. pylori status could be established accurately if two antral and two corpus biopsies were taken. Despite of the limitations in the present study, we tried to grade the histological parameters of further studies can be conducted to improve our findings by following the proper guidelines of the Sydney system. This study was performed in a single medical institution, which may not be a general representation of Nepal although the patients came from variety of ethnic groups and socioeconomic backgrounds. Our sample size is also less; thus recruitment of more patients may have improved the statistical power of this study.
The present study found strong correlation between H. pylori infection in gastric biopsies with neutrophilic activity and mononuclear cell infiltrate. Thus, pathologists should test for H. pylori if neutrophilic activity and mononuclear cell in filtrate are present in gastric biopsies.
We would like to thank Mr. Anil shah, the staff of histopathology department for his technical assistance.
SM: Conceived the idea, literature search, designed, analyzed data, and did manuscript writing and final approval of manuscript.
SR: Data collection, reviewed, editing and final approval of manuscript.
MT: Reviewed, literature search and final approval of manuscript.
AB: Did data collection, proof reading of manuscript and final approval of manuscript.
BPO: Review and final approval of manuscript.
PN: Proof reading and final review and approval of manuscript.


