Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
IKIMI Nathan Ukuoghene*1, Awotile Adenike Ololade2, Ashiwaju Modupe Olufunmilayo3, Enone Lilian Lami and Benjamin Oluwakemi2
Received: July 10, 2017; Published: July 20, 2017
Corresponding author: Nathan U Ikimi, Dental and Maxillofacial Department, State House Medical Centre, State House, Abuja FCT, Nigeria
DOI: 10.26717/BJSTR.2017.01.000211
Objective: The purpose of this study was to investigate the knowledge and practices of disinfection of dental impression in Lagos State University Teaching Hospital and Lagos University Teaching Hospital, Lagos Nigeria and also to find out if the practices of these two tertiary hospitals were up to acceptable standards.
Material and Methods: This is a cross-sectional descriptive study of dental health givers in the two hospitals and it was carried out using self-administered questionnaires to investigate the knowledge and practices of dental impression disinfection. Data was collated and analyzed with SPSS version 20.
Results: The questionnaires distributed were 270 and we had a response rate of 83.3 % (225 questionnaires were returned completed). Respondents with Bachelor of Dental Surgery degree were 118(52.4%) while 107 (47.6%) were final year dental students. Hand washing before wearing gloves was done by 208(92.4%); hand washing after making impression was practiced by 222(98.7%). Water and antimicrobial soap for hand washing was practiced by 109(48.6%); while 3(1.3%) made use of water without soap. Respondents who used 1% Sodium hypochlorite as choice disinfectant were 181(80.4%).
Discussion: This study revealed a sufficient level of awareness and practices of disinfection of dental impression in the two institutions. The reason for this could be the importance attached to infection control during the training of dental students. Continuous training and updating of knowledge on new dental impression disinfection techniques is stressed.
Conclusion: We can conclude at the end of this study that there is a good knowledge of dental impression disinfection among health givers in the two institutions studied and this is demonstrated by their regular practice.
Keywords: Antimicrobial Soap; Disinfectant; Infection control; Practices; Water
Abbreviations: FDI :Federation Dentaire Internationale; CDC: Centre for Disease Control; ADA: American Dental Association; EPA: Environmental Protection Agency; LUTH: Lagos University Teaching Hospital; LASUTH: Lagos State University Teaching Hospital; HCAI: Health Care Associated Infections
The oral cavity with the masticatory apparatus is a highly conducive environment for the growth and proliferation of bacterial [1]. Dentists and other dental care givers are at risk from cross-contamination from the mouth of patients and other infected working environment in the dental office [2,3]. According to the guidelines published by the British Dental Association, “the only safe approach to routine treatment is to assume that every patient may be a carrier of an infectious disease” [4]. In view of the above statement, standard precautions require that all dental health care workers should assume that the blood and body substances of all patients are potential sources of infection, regardless of the diagnosis, or suspected infection [5]. Dental workplaces are potential sites for the spread of various communicable and incurable diseases to, from and among patients and the dental staffs hence, cleaning and disinfection of dental impressions should be carried out before sending to the dental laboratory since a contaminated dental impression can spread infection in the dental laboratory [6]. Studies have shown that 67% of dental impressions sent to dental laboratories are contaminated by a host of different microorganisms [7] and some of these microorganism are known to have a survival rate of up to 5 hours on dental impression materials [7,8]. These microorganisms includes but are not limited to Streptococcus Species, Staphylococus Species, Escherichia Coli Species, Actinomyces Species, Anitratus Species, Pseudomonas Species, Enterobacter Species, Klebsiella Pneumonia and Candida Species [9].
The Federation Dentaire Internationale (FDI) requires as a standard precaution that all patients’ impressions should be rinsed under running water to remove saliva and visible blood. Then, they should be placed and sealed in a proper container and labeled as disinfected or not disinfected before being sent to the dental laboratory [10]. Also, the American Dental Association (ADA), the Centre for Disease Control (CDC) and Prevention with the Australian Dental Association, have all listed a similar standard procedure for the disinfection of dental impressions [11-13]. Nevertheless, care should be taken that the disinfection procedure does not have adverse effect on the dimensional stability or surface details of the dental impression. This is important because the various types of impression materials available have features such as: accuracy, elastic recovery, dimensional stability, flexibility, a long shelf-life, tolerable taste to patients and relatively affordable [14]. Thus, the choice of disinfectant depends on the type of impression material chosen since the composition, concentration of the disinfectant and the exposure time would greatly affect the compatibility of these disinfectants with the specific impression materials [15-18].
The ADA and CDC recommends the following disinfectants: 0.5% Chlorhexidine, 1% Sodium hypochlorite, 2% Glutaraldehyde and Iodine agents [11-13]. 1%Sodium hypochlorite has been generally accepted as the disinfecting agent of choice for alginate [19] and it is recommended by the Environmental Protection Agency (EPA) as a good surface disinfectant, with a non-irritating effect and efficacy against a wide variety of microorganisms; however, its unpleasant odor coupled with its considerable chemical instability is a major drawback [20]. Dental impressions should be decontaminated by immediate rinsing under running water and then completely immersed in a suitable disinfectant which exposes all the surfaces of the impression equally to the disinfectant or sprayed with the disinfectant which reduces the likelihood of distortion than immersion, before sending to the dental laboratory [21-23]. Tap water, used as pre-wash has been reported to reduce 40% to 90% of microorganism [22] and it also reduces the bacterial load, food debris, blood and saliva [11,13].
Studies carried out in Lahore Pakistan, reported that House officers and students had knowledge of infection control and were following the internationally acceptable standard procedures for dental impression disinfection [24]. However, a large number of dentists in Karachi Hospital in a different study where described as having “a poor knowledge about the use of disinfecting agents” [25]. None the less, a study in Rashi Irah reported that dentist there had a moderate knowledge of disinfection of dental impression materials but were not putting that knowledge to practice [26]. In a related study done among dental technologist at the dental laboratory of the Muhimbili National Hospital in Tanzania, out of the 1,453 dental impressions sent to the dental laboratory for a period of one year, there was no record or evidence of anyone of the impressions to have been chemically disinfected [27].
Therefore, since there is a potential danger of crosscontamination from dental impressionsbecause every patient may be a carrier of an infectious disease, it became imperative to carry out this study to investigate the knowledge and practice of disinfection of dental impression material among dental health givers in the dental centers of Lagos University Teaching Hospital (LUTH) and Lagos State University Teaching Hospital (LASUTH) in order to prevent a future epidemic of infectious disease sequel to cross-contamination from dental impression.
This is a cross-sectional descriptive study which was carried out among dental health givers in Lagos State University Teaching Hospital (LASUTH) and Lagos University Teaching Hospital (LUTH). We included dental practitioners who had actively taken dental impression for the previous 5 years, dentist in postgraduate residency training whose areas of specialization involved the taking of dental impressions and final year dental students. Excluded from this study were dentist in residency programs whose area of specialization did not involve the making of dental impression such as: Dental Public Health, Period ontology, Oral Medicine and Oral pathology. Also excluded were dental practitioners who had not taken impression for the previous 5 years, dental students who were not in the final year clinical class in dentistry, dental nurses and dental technologist. Dental nurses transport the impression to the dental laboratory where the dental technologist who are also not allowed to make impression, work on the cast. Dental consultants as a group were excluded, because a large number of them were not involved in impression making in the teaching hospital setting but rather, demonstration of impression making was done by the senior registrars and a few consultants, however, the role of the consultants was more of supervision. Application for ethical permit was approved by the research and ethics committee of the Lagos state university teaching hospital for the purpose of this research.
A modified [25] self-administered questionnaire was used as data collection tool to assess information on training institutions, qualifications and specialties. Without using any grading system to measure knowledge of respondents, the knowledge and practice of disinfection of dental impression materials was investigated using open and close ended questions. Respondents were asked questions such as “Do you wash your hands before using hand gloves” “Which is the most common disinfectant used in your institutions” and they were required to tick either “Yes” or “No” as answers to some questions; then choose the most appropriate option where various options were given, in other questions. The questionnaires were distributed and collected among the respondents by two of the researchers. Statistical analysis was done descriptively with SPSS 20 version (IBM Corp., Armonk, NY, USA) and presented as frequency tables. Chi-square was used to compare similarity in knowledge and practice between the two institutions.
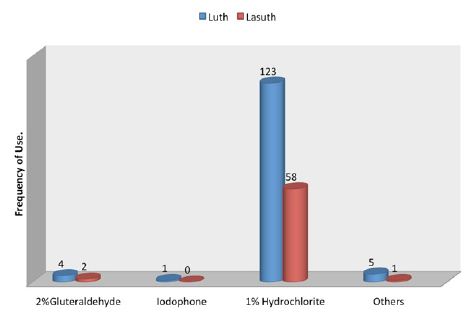
A total of 270 questionnaires were distributed in both Lagos University Teaching Hospital (LUTH) and Lagos State University Teaching Hospital (LASUTH) with a total of 225 questionnaires returned completed, giving a response rate of 83.3%. These were made up of 107 final year dental students, 24 dental surgeons in postgraduate residency training program and 94 dental house officers. Among the 225 subjects in both institutions were 127(56.4%) females and 98(43.6%) males, while a large number were qualified dental surgeons; 118(52.4%) had Bachelor of dental surgery (BDS) and 107 (47.6%) where yet to be certified Details of practices for preventing contamination of impression materials shown in (Table 1). Disinfection of dental stones is not the usual practice in both institutions. Details of Health givers disinfection practices shown in (Table 2). The knowledge of the best effective methods for disinfecting specific impression material was assessed. Details as shown in (Table 3). The disinfectants most commonly used were also investigated in these institutions. Details as shown in (Figure 1).
Table 1: Prevention of impression contamination.
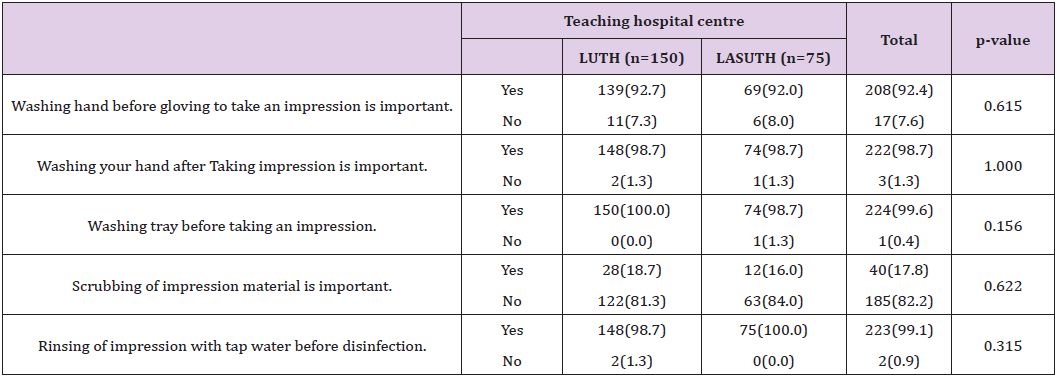
Table 2: Health givers disinfection practices.
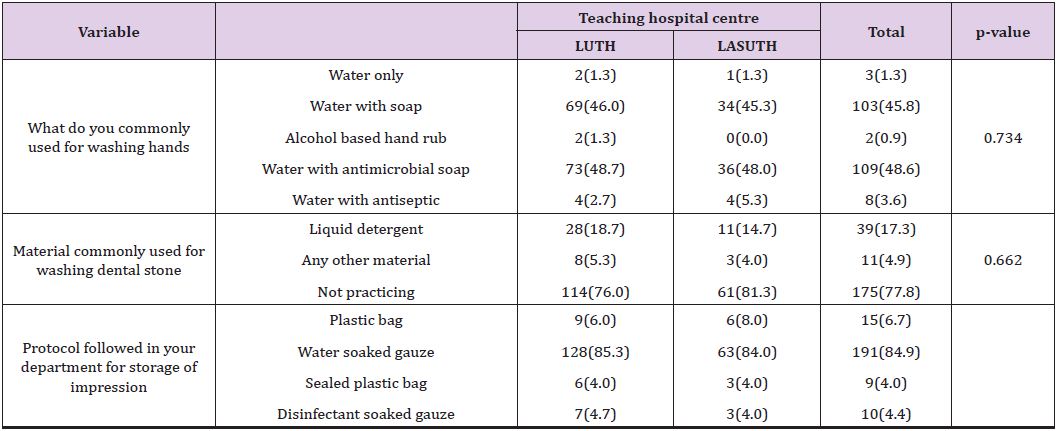
Table 3: Knowledge of various methods of disinfecting impression material.
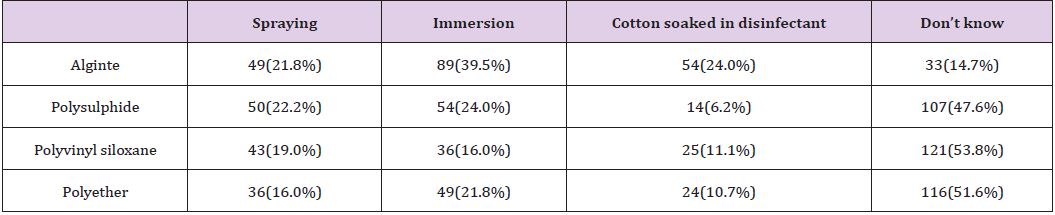
Figure 1: Disinfectants most commonly used.
In this present study, hand washing before wearing gloves was practiced actively and respondents were passionate about hand washing after making dental impression as shown by the 222(98.7%) that did hand washing after making dental impressions (Table 1). This practice is in consonance with the report that majority of the dentist from 9 dental colleges were practicing hand washing before and after making dental impressions in Karachi [25]. This is very important in the disinfection of dental impression to prevent crosscontamination since in recent times, there has been increasing evidence showing that hand hygiene is a very effective method by which health care associated infections (HCAIs) are reduced [28] and this was especially evident in Nigeria where increased hand hygiene both at household level and community level reduced the spread of the Ebola virus [29].
With respect to the care of impression trays, 224(99.6%) respondents were involved in washing impression trays before using them (Table 1). The impression trays in these two institutions, Lagos University Teaching Hospital (LUTH) and Lagos State University Teaching Hospital (LASUTH), consisted of mainly plastic trays. The usual practice is to wash the trays and sterilized them chemically by complete immersion in 1% Sodium Hypochlorite solution. Thus the rinsing done here is to wash off the taste of sodium hypochlorite from them. However, it has been reported in some dental institutions where metallic trays are often used that already autoclaved impression trays were being placed under running water before making impression [25]. After autoclaving metallic trays, the sterilization process is complete; passing them under running tap water would contaminate them again through water contaminants [23].
The practice of brushing off debris from impressions before being disinfected is not usually done in these two institutions and this is noticed from the large number that did not scrub the dental impressions (Table 1); only 40(17.8%) respondents practice scrubbing of dental impressions. This low level of practice of scrubbing dental impression is similar to the practice in the United Kingdom where it is reported that only 2.6% registered dentists were brushing debris from impressions [30]. On the other hand, while washing of impressions under running tap water following removal from the mouth was done by most of the respondents in this present study (Table 1), Almortadi and Chadwick reported that just only 37.2% of dental health care providers in their study, washed impressions under running water [21]. The practice of washing dental impressions after removal from the patient’s mouth is in agreement with the American dental association guidelines that recommends that impression should be washed under running water when they are removed from the mouth of a patient as part of decontamination to reduce food debris, blood and bacteria load on the impression [11,13]. Assessing the knowledge of dentist on the various types of disinfectants, Amin F et al reported that 43% of health workers were found to have insufficient knowledge of the best disinfectant for alginate impression material [25]. In this present study however, 181(80.4%) respondents use 1% sodium hypochlorite (NaOCl) as the most common disinfectant for alginate impression material (Figure 1) and this agrees with report that alginate maintains its dimensional stability after being soaked in 1% NaOCl [19]. Moreover, alginate impression material is also cost effective and readily available.
Regarding the protocol followed for storage of dental impression, Zaker-Jafare et alreported that dental impressions sent to dental laboratories where not properly packaged by dental students [26]. In this present study, dental impressions where covered with gauze soaked in water after being disinfected (Table 2) and it is not the practice to send information as to the type of disinfection that has been done. This is contrary to the current guidance recommended by the British Dental Association [30] which states that “the responsibility for ensuring impressions have been cleaned and disinfected before dispatch to the laboratory lies solely with the dentist. It is good practice to agree the cleaning and disinfection process with the laboratory and label (italics mine) the device to indicate disinfected status.” When labeling is done, the dental technologist would know whether to disinfect an impression which has not been disinfected or not because subjecting already disinfected dental impressions to chemical disinfectants might distort fine details of the impression.
While it is believed that complete immersion of dental impression is likely to expose the entire surface to disinfection more that spraying, respondents in this present study had little knowledge of the best methods for disinfecting polysulphide, polyvinyl siloxane and polyether impression materials (Table 3). Gladwin and Bagby noted that using spray or immersion methods for 30minutes resulted in the dimensional change of these impression materials at < 0.5% (less than 0.5%) which was not clinically significant according to the American Dental Association specification criteria. However when immersed in 1% NaOCl for more than 60 minutes, polyether had “significantly” higher dimensional variation [19]. This study recommends that it is essential for written information on disinfection process should be sent with the dental impression to the dental laboratory; it is also important to monitor and maintain the high standard of disinfection of impression material in these two dental schools. Written information would show the chemical disinfectant that was used and the method of disinfection that was done [10] thus the dental laboratory technologist would be aware if disinfection of impression material was carried out or not by the dentist.
Within the limitations and methodology of this study carried out in the Lagos University Teaching Hospital (LUTH) and the Lagos State University Teaching Hospital (LASUTH), it can be concluded that both institutions have a similar knowledge and practice of disinfection of impression material which can be described as being sufficient since it conforms to international standards. This should be sustained through awareness programs such as dental updates, seminars and workshops. Furthermore, researches such as this should be carried in other regions of Nigeria to have a comprehensive picture of the knowledge and practices of disinfection of impression materials in Dental training institutions. The results from such studies can be used as tools for advocacy on sponsorships of more training programs, update lectures and regular workshops on recent disinfection techniques and maintaining uniform high standards of infection control in Dental training Institutions.
I wish to acknowledge the contributions of the following person
A. Mrs Roselyn Ikimi for proof read the entire manuscript.
B. Mr Clement Akinsola, the statistician who did all the statistics.


