Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Daniel Ramos Pires*
Received: July 20, 2017; Published: July 25, 2017
Corresponding author: Daniel Ramos Pires, Cirurgião ortopédico no Hospital Terra Quente - Hospital Terra Quente, Portugal
DOI: 10.26717/BJSTR.2017.01.000223
We describe a rare case of a Candida albicans PJI (prosthetic joint infection) in an otherwise healthy woman treated with a two-stage revision and directed antifungal therapy. One year after the final surgery, the patient is doing well with a good clinical and functional result. This type of diagnosis is difficult and often miss interpreted needing a high level of clinical suspicion for its proper management.
Keywords: Candida Albicans; Prosthetic Joint Infection; Two-Stage Revision; Anti Fungal Therapy; Antibiotic- Loaded Bone Cement
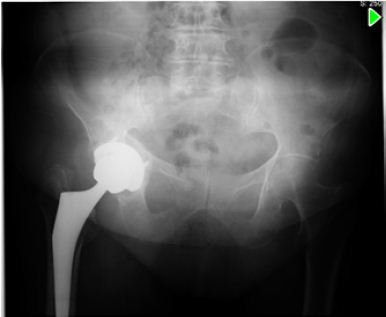
A 79 years old woman, otherwise healthy was submitted to a right THA for primary arthrosis (Figure 1). 3 weeks after surgery, she presented with hip pain and serous drainage from the surgical wound. She was therefore submitted to surgical debridement and substitution of polyethylene component. Cultures were taken (sterile) and empirical antibiotic therapy with vancomicin (2g/day) and rifampicin (600mg/day) was initiated.
Figure 1
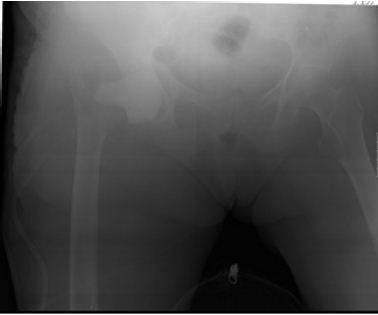
Because of persistent pain and drainage, the patient was submitted to implant removal and placement of a static cement spacer impregnated with gentamicin (Figure 2). The cultures taken at the time of the second surgery isolated Candida albicans. In light of this new evidence fluconazole (400 mg/day) was added to the previous scheme with good clinical evolution. The patient was discharged to a nursery home after 2 weeks of intravenous treatment, maintaining oral treatment with fluconazole (400mg/ day), weight bearing as tolerated.
Figure 2
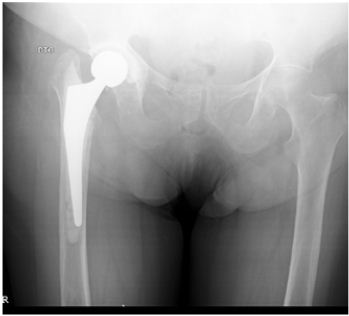
After 9 months of follow-up,with good clinical and analytic evolution, and accordingly to patient desire, the second stage of the treatment – THA revision – was performed (Figure 3). New cultures were taken at this procedure that came back negative. Post-operatively, there was a positive clinical evolution, without any further complications. After being discharged, the patient followed a 6 months scheme of oral prophylactic therapy with 400 mg/day of oral fluconazole, and was tightly monitored until completing 1 year post-surgery, being quite pleased with the end result, from the functional point of view.
Figure 3
Fungal PJI is uncommon, with a global incidence currently unknown, but estimated as about 1% of all PJI [1-5]. Most fungal PJIs are caused by Candida albicans and Candida parapsilosis [4]. Extensive comorbidity and decreased immunity are considered risk factors for fungal infections and are associated with a poor outcome [1,4,6,7]. Immuno suppression, malignancy and/or the use of anti neoplastic agents, drug abuse, prolonged use of antibiotics, presence of indwelling catheters (intravenous, urinary or parenteral alimentation), diabetes mellitus, malnutrition, rheumatoid arthritis, history of multiple abdominal surgeries, severe burns, tuberculosis, and previous bacterial infection of the prosthesis are some of the predisposing host factors [7].
The diagnosis of a fungal PJI is difficult: its mechanism and clinical features often mimic that of chronic bacterial infection, with an indolent onset, and most often patients present with swelling and pain without other symptoms of infection [4,8-10]. On the other hand, culture results may be interpreted as contamination, and most authors recommend obtaining multiple samples, prolonged culture, and special staining [4]. In specific cases one should expand diagnostic testing to include tissue samples for histological examination, especially in cases where there is a high index of clinical suspicion [7].
In our case, the patient presented with an acute PJI infection, being the diagnosis delayed because of culture results. According with the latest consensus on PJI infection [11], she was treated with surgical debridement and antibiotics. Several different treatment methods, including antifungal drugs, debridement with retained prosthesis, resection arthroplasty, and two-stage exchange arthroplasty have been reported, with variable outcomes [1,5,11]. Although a two-stage revision was preferred by most surgeons, with the highest success rate (85%) [5], controversy still exist with regard to the ideal interval between implant removal and reimplantation, the usefulness of antifungal-loaded cement spacers and the duration of systemic antifungal treatment [2,5,7,9].
A long period of oral antifungal treatment has been recognized as being an essential factor for the success of staged reimplantation after a fungal prosthetic joint infection. However, the required duration of treatment is unclear: the literature shows a mean of 4,8 months with a range from 1 week to 1.5 years [4,5]. Some authors have suggested a 3-month period [4] whereas others have advised reimplantation only when repeated (aspiration) cultures are negative [4]. The Infectious Diseases Society of America recommends treatment with fluconazole or amphotericin B for at least 6 weeks after removal of the arthroplasty in most patients with fungal PJI [2,4-7,12]. A review of the literature shows that duration (comparing 6 weeks and 3 months of antifungal treatment) does not appear to influence outcome after reimplantation [4,5].
Reimplantation may be done after successful eradication of the infection, as defined by the lack of symptoms after the suspension of antifungal therapy [2]. Because there is no specific laboratory test to monitor fungal PJI, the ones used for monitor bacterial infection may also be used in this setting [7]. Repeated aspiration prior to reimplantation may help the surgeon determine timing of surgery [7]. After reimplantation some recommend a minimum of 6 weeks to a maximum of 6 months of oral fluconazole therapy [1,9], although there isn´t good quality data to support this recommendation [7].
In the case under discussion, we opted for a longer antifungal course (6 months) because normalization of the analytical parameters (C reactive protein and erythrocyte sedimentation rate) took longer than anticipated.
Recurrent infection after delayed reimplantation is a serious complication and often results in permanent removal of the joint prosthesis. The recurrence rate after 2-stage reimplantation has been described as being up to 33% [2,4-6,13].
If removal of components is not an option, chronic suppression therapy with fluconazole is recommended [4].
Amphotericin B or fluconazole have been considered the drugs of choice for treatment of fungal infections [2,4,6,9]. Fluconazole has a high oral bioavailability and good bone and synovial penetration capacity [1,2]. Amphotericin B is one of the most toxic antimicrobial drugs, with a high incidence of adverse effects [4,6]. On the other hand, primary resistance against fluconazole is common in some non-albicans candida species [4] and so susceptibility testing may be required when resistance to fluconazole is suspected [7]. The use of echocandins was only described in a few reports [4], but it may be a good alternative- due to its low toxicity and broad spectrum- especially for fluconazole-resistant fungal species, or if the patient does not tolerate amphotericin B. However, the longterm side effects are unclear [4,6].
The introduction of antibiotic-loaded bone cement improved the results of revision arthroplasty for bacterial sepsis, and it is now considered the gold standard for the treatment of bacterial prosthetic joint infections [1,5] However, the efficacy of antifungalloaded cement spacers in the treatment of fungal prosthetic joint infections is controversial [6,13]. Theoretically, amphotericin B seems to be an ideal agent to be mixed with bone cement because of its heat stability, broad antimicrobial spectrum, and availability in powder form [5,7,13,14]. When voriconazole is chosen, loss of mechanical strength should be kept in mind when fabricating spacers [7].
Some recent in vitro studies document a very good elution of voriconazole from polymethyl methacrylate (PMMA) and activity on bioassay, but its presence in bone cement leads to loss of compressive strength. On the other hand, it is now being questioned which dose of antifungal drugs in bone cement is high enough to destroy fungal bio films [9]. Facing the fact that fungal PJI represents a serious increasing problem in arthroplastic surgery of the hip, there is an urgent need to establish guidelines for their treatment [9].


