Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
DC Roy* and R Gogoi
Received: July 21, 2017; Published: August 03, 2017
Corresponding author: Dr. DC Roy, PhD, FASc, Prof cum PI, Department of Pharmacology & Toxicology, C.V.Sc, Khanapara, Guwahati-781022, Assam,India
DOI: 10.26717/BJSTR.2017.01.000250
The study was undertaken to monitor marketed pork of Assam and its neighboring states Meghalaya and Manipur for Fenbendazole residues using Ultra High Performance Liquid Chromatography (UHPLC). 675 Samples of marketed pork of Assam, Meghalaya and Manipur were collected for the study. The samples after collection were preserved at -20 C. Recoveries of Fenbendazole in pork ranged from 89-98 %.Out of the tested samples, 29 samples were detected to be positive for trace residues of Fenbendazole which were well below the Maximum Residue Limit (MRL) value.
Keywords: Fenbendazole; Monitoring; Pork; Residues; UHPLC
Residues of Veterinary drugs are one of the major problems for food contamination. Veterinary drugs residues usually accumulate in the liver or kidney rather than other tissues. Fenbendazole is an anthelmintic frequently used in farm animals to treat parasitic infestations in pig [1]. The most likely reasons for Fenbendazole residues may result from human management, such as improper usage, including extra label or illegal drug applications [2].
The North-Eastern states of India is characterized by a high proportion of tribal people for whom pig rearing is integral to their way of life and pig meat is considered as an important food item. Thus the present study was undertaken for monitoring of Fenbendazole residues in pork samples from various areas of Assam and its neighboring states Meghalaya and Manipur using UHPLC.
675 samples of swine tissues comprising of Muscle, Kidney and Liver were collected from different markets of Assam, Meghalaya and Manipur (Table 1). Representative samples weighing 30 g each belonging to same carcass were wrapped in polythene bags and transported in thermo-cooled containers jacketed with ice. The samples were stored at -20 C till the time of processing. The levels of Fenbendazole were determined using a UHPLC system of Make: Dionex with a wavelength at 290 nm as per Method described by Cooper et al. [3]. The samples were separated on a RP-C 18 column and were eluted with a mobile phase of mixture of Water and Acetonitrile in the ratio of 70:30 v/v. The isocratic mode was run at a flow rate of 1 ml/min.
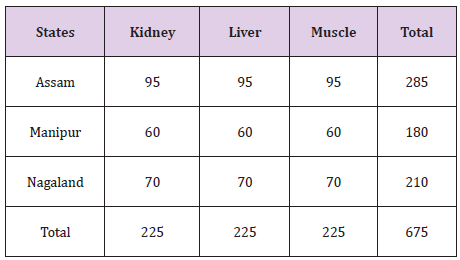
Table 1:Collection of Pork Samples.
Fenbendazole standard (Dr Ehrenstofer, Germany; HPLC grade Acetonitrile (Fisher), Methanol (Fisher); other chemicals and solvents of analytical grade and HPLC grade water (Fisher) were used for the study.
The fascia and fat of pork were removed and then cut into small pieces.5 g of the sample was taken in a glass test tube and to it added 2ml of Phosphate buffer and mixed. The mixer was then sonicated by setting at 15 micron amplitude for 20 cycles with a stop time of 30 second interval at low temperature which was maintained with crushed ice. The sonicated sample was then left undisturbed for 15 mins for allowing the extract to dissolve in the solvent. The sample was then centrifuged at 10,000 rpm at O centrigate in a refrigerated centrifuge machine. The supernatant was separated and filtered through a Whatman filter paper no 42.Clean up of the extract was done by using C18 Solid Phase Extraction (SPE) method and then finally eluted with 5 ml of methanol. The extract so obtained was filtered through a Millipore filter paper (0.22um).The eluted sample 20ul was then injected into the UHPLC system for analysis.
Linear calibration curve of Fenbendazole having correlation coefficient (R2) of 0.99 was achieved.Recoveries of Fenbendazole in pork ranged from 89-98 % .This was similar to that reported by Cooper et al. [4]. Fenbendazole was eluted under isocratic conditions. The excitation/emission wavelengths use for UV detection were 290 nm for Fenbendazole [5-8]. The separation of the analyte was achieved in less than 5 mins. Acetonitrile was effective in the deprotenization of pork samples and in the isolation of analyte from spiked samples. This method allows the determination of residues of Fenbendazole in different matrices with higher sensitivity.
Overall, nine zones within the state of Assam and five zones from the states of Meghalaya and Manipur have been selected and a total of 675 samples of pork comprising of muscle, liver and kidney were collected. A total of 29 pork samples were detected to be positive of Fenbendazole residue. As listed in Table 2, only 14 kidney, 10 liver and 5 muscle samples showed detectable Fenbendazole residues using UHPLC. All the samples were below the permissible limit. Residue level of Fenbendazole detected in muscle, kidney and liver were 0.029-0.075 ug/g, 0.013-0.087 ug/g and 0.010-0.0426 ug/g respectively (Table 2).
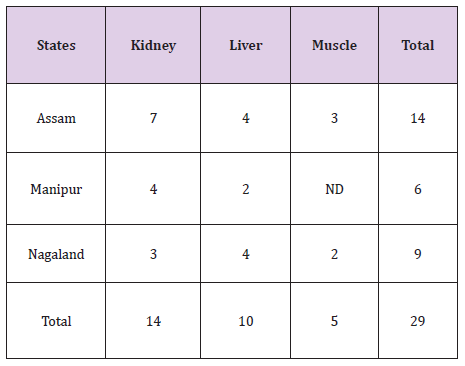
Table 2:Location wise Distribution of Fenbendazole Residues in Pork Samples.
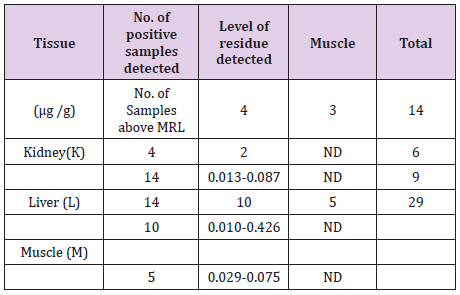
Table 3:Fenbendazole Residue Level in Swine Tissues.
A total of 14 (fourteen) samples from Assam, 9(nine) samples from Meghalaya and 6(six) samples of pork from the state of Manipur were detected to be positive of residues of Fenbendazole as given (Table 3). From the state of Assam recorded the maximum number of positive samples followed by Meghalaya and Manipur respectively [9,10].
675 samples of pork were collected from different pork market of Assam, Meghalaya and Manipur. Out of the screened samples, 29 samples were detected to be positive for trace residues of Fenbendazole using UHPLC which were all below the MRL level.
The authors acknowledge the financial assistance and help received from ICAR, New Delhi and AAU, Jorhat.


