Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Liliane Pereira, Claudio A Téllez S*, Priscila Fávero and Airton A Martin
Received: July 28, 2017; Published: August 09, 2017
Corresponding author: Claudio A Téllez S, Biomedical Engineering Innovation Center, Biomedical Vibrational Spectroscopy Group. Universidade Brasil - UnBr - Rua Carolina Fonseca, Itaquera São Paulo/SP, Brazil
DOI: 10.26717/BJSTR.2017.01.0002263
The process of glycation is a non - enzymatic reaction between sugar and free amino group of proteins, leading to skin aging. In this sense, the present study aimed to analyze the changes resulting from glycation in the dermis by Confocal Raman spectroscopy. The analysis was performed with 30 patients with skin types I and II, divided into 3 groups: 10 healthy young women aged 20-33 years, 10 healthy elderly women and 10 elderly women with diabetes type I and II in the age group of 65-80 years. Raman spectra were collected from 70-130 microns in depth and the data were analyzed. It was observed that the amide I band was centered at 1664 cm-1 for the young participants and healthy elderly which may be due to the presence of water and change in the direction of the shorter wavelength was observed which is centered around 1662 cm-1. Considerable changes were observed for the elderly participants with diabetes and the peak was centered around 1658 cm-1. Thus, it is inferred that hyperglycemia in elderly subjects with diabetes mellitus accelerate the formation of advanced glycation end products, which increase the type I collagen degradation in human skin.
Keywords: Confocal Raman spectroscopy; Glycation; Aging; Collagen; Skin; AGEs; Amide I
The effects of the glycation reaction in the skin`s proteins at cellular level accelerate the aging process and results in wrinkling and stiffness. Without the action of an enzyme, protein reacts with reducing sugar and [1-4] give rise to Advanced Glycation Endproducts (AGEs). These AGEs cause degradation of type I collagen and apoptosis of fibroblasts, resulting in alterations of the amide I bands which correspond to the secondary structure of this protein [5-12]. The Confocal Raman spectroscopy is a non invasive technique which uses a laser of 785 nm as an excitation source, with the ability to measure the biochemical composition of the skin, without causing damage and does not need the preparation of material for analysis [13-17]. By the objective lens of the inverted microscope, the laser is guided to the skin and collects the inelastic scattering of light in real time and at different depth profile [17-22] making it a suitable tool to probe human skin. Therefore, the main goal of this study is to characterize and compare the chronological effect of skin aging due to the process of glycation in amide I of type I collagen in the dermis of healthy young women (HYW), health elderly women (HEW) and diabetic elderly women (DEW).
Confocal Raman setup for in vivo human skin experiments were performed by a confocal Raman system from Rivers Diagnostics® (Model 3510 Skin Composition Analyzer) coupled to Laser light of 785 nm and is focused into the skin by a microscope objective. The power of the laser at the skin was set as 27 mW. The laser light was focused on the forearm skin with an objective of the microscope (40x) located under the quartz window. The Raman signal was collected by a CCD camera and recorded by a computer coupled to the system. The data were obtained in the fingerprint region (400- 1800 cm-1) from 70-130 microns below the skin surface with a spatial resolution of 3 microns, integration time of 60 seconds and 2 accumulations. Pre processing was done in the order normalization of the amide I band, smoothing and baseline correction using LabSpec 5 software.
This study has been approved by the ethical committee and participants with skin type I and II were selected, according to Fitzpatrick classification [18]. The participants with the presence or history of skin diseases, irritation, sensitivity to cosmetic products, pregnancy, lactation and use of moisturizer in the past 48 hours before analysis were excluded. A total of 30 women participants were selected and divided into 3 groups: 10 volunteers HYW, 20- 33 years old, 10 DEW type II, 65–80 years old and 10 HEW, 65–80 years old. Noninvasive instrumental technique used in the research presents no risk, cause no damage to the individual participant.Before starting the skin assessments, participants remained in air-conditioned environment at 23 ± 2°C and relative humidity of about 51 ± 5%, for approximately 60 minutes. The subjects were positioned with the forearm in an aluminum plate, containing a quartz optical window. Before collection of Raman spectra, the skin was cleaned with cotton soaked in 1.0 ml of 70% ethyl alcohol.
Confocal Raman spectroscopy technique enables chemically selective and non-destructive sample analysis with high spatial resolution in three dimensions [23-28]. By this instrument we can obtain knowledge concerning the secondary structure in the type I collagen, with wavenumbers centered at 1590-1720 cm-1 which corresponds to amide I and with major contribution of v(C = O). According to Maiti et al. [28] & Nguyen et al. [29] the wavenumber of 1655 cm-1 corresponds to triple helix structure of protein. Peptide bonds of proteins through the C = O stretching vibration can be detected in the amide I position which corresponds to the triple helical structure of the protein in young skin tissue collagen [24-38]. However, in our research, a curve fitting method for Raman data was adopted for a more detailed analysis of the secondary structure of proteins. Deconvolution of Raman spectra of all 30 women participants was performed to seek the changes of amide I in type I collagen among the three groups. The constituent bands of amide I have been chosen from the second order derivative after applying 5 points filter and a total of 10 specific bands were obtained for young participants in the spectral range 1500-1720 cm-1.
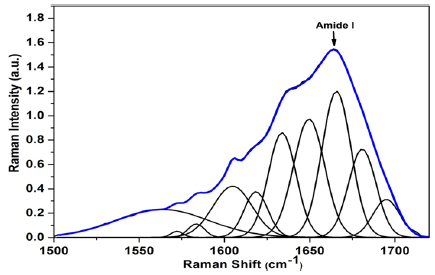
The alpha helix structure is centered at wave number 1655 cm-1 [24,38]. Gniadeka et al. [27] reported that peptide bonds of proteins through the stretching vibration C=O amide I corresponds to the helical structure of the protein in young skin tissue collagen [27]. In the HYW, AGEs markers were not found and the bands were centered around 1564, 1572 and 1618 cm-1 which corresponds to Tryptophan, 1583 and 1606 cm-1 maybe attributed to Phenylalanine and the amide I band centered around 1634, 1650, 1664, 1681 and 1695 cm-1 (Figure 1).
Figure 1:Average of the deconvoluted spectra of amide I in type I collagen of young participants.
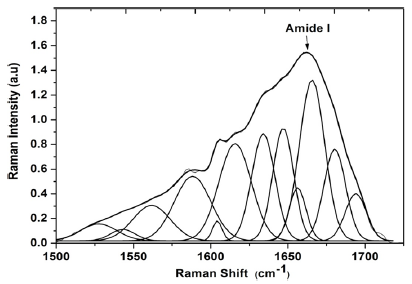
Twelve specific bands were obtained for the HEW in the spectral region of 1500- 1720 cm-1. For these volunteers, a single AGE marker centered at 1543 cm-1 was located by the calculation of the Density Functional Theory (DFT) which corresponds to pentosidine. Bands were found at 1526 cm-1 which corresponds to v(C = C), bands at 1562 and 1616 cm-1 were assigned to tryptophan, 1586 and 1604 cm-1 may be assigned to amino acid and phenylalanine, respectively. Bands at 1634, 1648, 1656, 1662, 1680 and 1693 cm-1 corresponds to amide I [19,23,32] (Figure 2).
Figure 2:Average of the deconvoluted spectra of amide I in type I collagen of healthy elderly women.
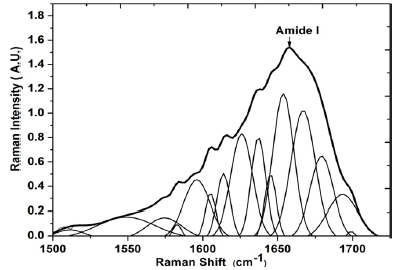
Fifteen specific bands in the spectral range 1500 to 1720 cm-1 were selected for the HEW participants. For this group, two markers of AGEs were located. The band centered around 1511 cm-1 correponds to AGE glucosepane and the band at 1700cm-1 corresponds to AGE pentosidine. The pentosidine and glucosepane are that most affected AGEs makers in human dermis and in patients with hyperglycemia, the amount of these AGEs is twice that of healthy individuals [19,31-36] which is in agreement with the results obtained from our early study. Also it was found the bands centered at 1549, 1573 and 1626 cm-1 corresponds to tryptophan, 1582 cm-1 and 1604 cm-1 assigned to phenylalanine and the amide I band located at 1638, 1645, 1658, 1668, 1678 and 1695 cm-1 [1,23,30] (Figure 3).
Figure 3:Average of the deconvoluted spectra of amide I in type I collagen of elderly women with diabetes type I and II.
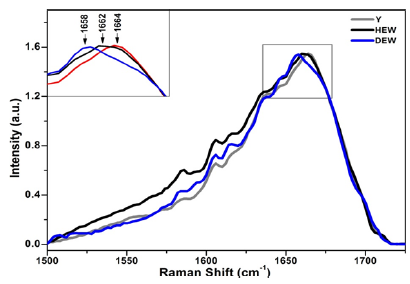
From the results, it was observed that the bands of amide I demonstrate predominance of alpha helix structure [15] and shift in the wave number was observed among the three groups. Zhang et al. [25] and Nguyen et al. [26] reported that the spectral profile of the amide I band change when the relative humidity rises [24,25]. There was also a shift in the wave number of the amide I band of 1672 and 1665 cm-1, attributed to stretching vibration of (C = O) in collagen. The Raman band centered at 1664 cm-1 corresponds to amide I in young healthy participants (Y) participants shifted to the region of shorter wavelength, in HEW participants in the spectral region of 1662 cm-1 and in DEW there was a greater displacement for the spectral region 1658 cm-1 (Figure 4). The obtained information can be justified by the lower wave number region which determines the reduction of the force and energy of the chemical bonds of the molecules, since the frequency is proportional to the energy of links [15,25]. Water plays a crucial role in maintaining the conformation of the native collagen molecule [24,26,28]. In HEW, there is a degradation of type I collagen, because of the chronological aging and the presence of water molecules in triple helix. In group DEW, in addition to the chronological aging of human skin, excess sugar in the fibers, presence of AGEs, a further displacement was observed. Therefore, the chemical bonds of the molecule in these participants have less binding force of its atoms and lower power, because of no degradation of its structure by sugar or AGEs. Gniadecka et al. [28] reported that the protein/water reduces in the chronologically aged and photoaged skin, which results in a significant shift of the amide I band position for the lower frequencies when compared with Y participants [26-28]. This change is directly linked to the reduction in collagen stability, proposing changes in the conformation of the structure of protein in the chronologically aged skin tissue.
Figure 4:Averaged and normalized spectra of Amide I of type I collagen of the three research groups: Young healthy participants (Y), Health Elderly Women (HEW) and Diabetic Elderly Women (DEW). Highlighted region (1500- 1720 cm-1) corresponding to amide I of type I collagen.
The observed spectral shift of shorter wavelength in older participants with diabetes, can be explained due to high degree of protein folding, which becomes more rigid, compact and low stable. According to Zhang et al., 2011, it was concluded that the band centered at wavenumber 1668 cm-1 is linked to vibration of (C = O) stretching, with a carbonyl group, where, only one water molecule is involved. With increasing relative humidity, this vibration presents a deviation in the 1672 cm-1 and 1665 cm-1 and the intensity reduces extensively [24,25]. According to Nguyen et al. [29] the band centered at 1658 cm-1 which corresponds to hydrogen bonds in the (C = O) stretching vibration, where two water molecules react with a carbonyl group [24,28]. With the increase in relative humidity, the wavenumber shifts to lower frequency regions, because the carbonyl groups are likely to be involved in hydrogen bonds with two water molecules instead of only one. This can result in a change in the compression of the collagen fiber bundles due to chronological aging and aging process due to the glycation of proteins. The bands in the spectral range 3350 cm-1 and 3550 cm-1 comprise the (O – H) vibrations of the free water molecules. However, for analyzing the stability of collagen and hydration, it is necessary to quantify the water molecules and can be linked by analyzing vibrations in 1658 and 1668 cm-1 which are assigned to the (C = O) bond of collagen. According to research performed by Maiti et al. [24] and Zhang et al. [25] hydrogen bonding occurs with one of the atoms in a stretching vibration thereby decreasing the frequency which can be correlated to our results [25,29]. Hence, we conclude that, an overall reduction in the composition of secondary structure alpha helix for healthy elderly volunteers and also on a larger scale for DEW was observed because of the accelerated advanced glycation end products.
The present study shows that Raman spectroscopy is a promising tool to investigate bio-physiological mechanisms, their effect on the chronological aging and the glycation process in the human dermis. This technique has the advantages of In vivo analysis, real time, non invasive and no sample preparation. For health elderly women group, a single AGE marker was located at 1543 cm-1, which corresponds to AGEs pentosidine. For DEW, two AGEs markers were found at 1511 cm-1 and 1700cm-1 which correponds to AGEs glucosepane and pentosidine respectively. The differences in water content in the dermal collagen were correlated by the shift in Raman intensity of amide I at 1664 cm-1 for HYW and at 1662 cm-1 for HEW. These bands are attributed to the stretching vibration mode (C=O) of the secondary structure of the collagen chain and are sensitive to presence of water molecules in the interstitial space of aged collagen. Less water content was found in the DEW than HEW and this can be explained by the hyperglycemia of the DEW volunteers. Therefore, the ability of Raman spectroscopy was demonstrated to investigate the structural changes of collagen in the reticular dermis caused by chronological aging and the glycation process.
The authors acknowledges the Brazilian agencies for financial grant vide projects: CNPq (307809/2013-7), CAPES (88881062862/2014-01), FAPESP (2012/21904-2) and FINEP.


