Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
DM Allon1*, Irit Allon2, Ilana Kaplan3 and Dan Guttman4
Received: July 20, 2017; Published: August 14, 2017
Corresponding author: D M Allon, Department of Oral and Maxillofacial Surgery, Rabin Medical Center, Petach Tikva 49100, Israel
DOI: 10.26717/BJSTR.2017.01.000269
SSC: Squamous Cell Carcinomas; EMT: Epithelial-Mesnchymal Transition; PDGF: Platelet- Derived Growth Factor; SCF: Stem Cell Factor; HPF: High-Power –Fields; SMA: Smooth Muscle Antigen; PDGF: Platelet-Derived Growth Factor; GIST: Gastrointestinal Stromal Tumors
Keywords: Cerebral Palsy; Parents; Children; Adolescents; Quality Of Life; Physiotherapy
Over 90% of head and neck cancers are squamous cell carcinomas (SCC) which are the most common malignancies in the oral cavity. In spite of improved therapeutic procedures, SCCs generally exhibit poor prognosis and in addition, treatment results in significant functional and cosmetic defects. Several factors contribute to a poor prognosis for upper aerodigestive tract SCC patients including the delayed detection of cancerous lesions and the tendency to develop multifocal malignancy and premalignant lesions as a consequence of field cancerization. It has been generally accepted that prompt detection of early oral cancer or epithelial dysplasia is required to improve prognosis. Therefore it is important to identify markers for carcinoma progression.
As with other cancers, most deaths of oral cancer, amongst them tongue cancers with greatest prevalence, result from local invasion and distant metastases. The landmark of carcinoma progression during the invasive and metastatic phases is epithelial cell plasticity and dedifferentiation, which is similar to epithelial-mesnchymal transition (EMT) that occurs during embryonic development. EMT is the process that cells undergo to switch from a polarized epithelial phenotype to a motile mesenchymal phenotype. This process can occur during embryonic development, wound healing, fibrosis and cancer progression and has extensively reviewed in the last ten years (kalluri and Nielsen 2003, thiery 2003, schiller 2004,thiery and sleeman2006) [1-3]. Loss of epithelial cell polarity and acquisition of motility results from the disappearance of cell junction adherence molecules, reorganization of cytoskeleton and redistribution of organelles (thiery and sleeman2006). Uncovering the mechanism for EMTs is one strategy to predict tumor progression and possibly develop therapeutic intervention. This is complicated by the diversity of molecular mechanisms contributing to the plasticity of epithelial cells in different tissues (gotzmann 2004) [4]. Several signal transduction pathways emerged as important for EMT, such as autocrine factors: EGF, HGF Insulinlike growth factor, platelet –derived growth factor, Want signaling, notch signaling, and Hedgehog signaling. These pathways are also controlled by crosstalk between each other and with RTK/Ras and TGF-b/bone morphogenic protein signaling.
CD-117 is one of the trans membrane receptor tyrosine kinases is, a 145 kd4 protein, encoded by the c-kit proto-oncogene [5]. It is the receptor of platelet- derived growth factor (PDGF) and is structurally identical to stem cell factor (SCF). Some authors refer to the ligand as steel factor [6-13]. Not only is it normally expressed in a variety of human tissues, including breast epithelium, germ cells, melanocytes, immature myeloid cells, and mast cells, the over expression of c-kit has been reported in extensive immuno histochemical studies of several neoplasms [14-31]. Nonetheless, a limited amount of studies aimed to investigate the expression of c-kit in SCC of the upper aero-digestive tract, oral mucosa, namely tongue.
The purposes of this pilot study were to preliminarily evaluate c-kit expression in the neoplastic cells of squamous cell carcinoma of the tongue (TSCC), to learn whether this possible expression is related to a special location within the epithelium or to a different cell morphology and to inquire whether there is a trend towards a clinic pathological correlation, specifically patient outcome after 5 years.
The study was approved by the institutional Helsinki committee. The study sample consisted of 17 patients with primary TSCC; all were treated at the Departments of Oral and Maxillofacial Surgery and Otorhinolaryngology of a single major tertiary center from January 1990 to December 2004. Patient TNM data are elaborated in (Table 1). Clinical and demographic data were obtained from patients’ files. They were divided into two groups according to the clinical outcome: the first group, consisting of seven patients, where no evidence of disease was recorded in their post operative and oncological follow up documentation 5 years post operatively; and the second group, with the other ten patients that died of the disease.
Formalin-fixed, paraffin-embedded tissue resection blocks were retrieved from the archives. 5 μm sections were submitted to immuno histochemistry. Briefly, sections were pre-treated by microwave heating for 3 times, 5 minutes each, in 10 mm sodiumcitrate buffer at 600W. A monoclonal rabbit antibody for CD- 117(c-kit, Dako, Hamburg, Germany, dilution 1:100) was applied, and counterstained by diaminobenzydine. The sections were rehydrated and then immersed in 10 mM sodium-citrate buffer in a microwave oven, 3 times for 5 minutes each. The evaluation of the slides was performed using a light microscope which at X400 magnification, in a random order, by single investigator, blinded to the and case histories. We used Gastro Intestinal Stromal Tumors as positive controls.
The entire tumor thickness and adjacent stroma were examined for c-kit positive cells. Location and morphology were recorded as well. Location: stroma, invasive front, middle, upper. Morphology: polar, spindle/fibro blastoid. In each slide, the total number of positive cells as well as the number of CD-117-positive cells was recorded in 5 consequent high-power –fields (HPF) at x400 magnification. A mean labeling index was calculated for each case. Positive CD-117 staining was considered cyto plasmatic. We avoided stromal mast cells false positive CD-117 staining both by counting only spindle cells and by excluding cells that exhibited fine cytoplasmic granulation.
Difference between the study and control group was done using the non- parametric Mann-Whitney U test. Significance was determined with P<0.001.
The study group consisted of 8 male (47%) and 9 female (53%) patients aged 26 to 88 years at diagnosis (mean 54.8 years+- 17.9). One had a history of alcohol consumption and 6 (35.3%) were heavy smokers (>10 pack-years). Tumor staging was done according to the WHO TNM classification system. Five patients (34.8%) had T1 disease; 11 (64.7%) T2; and 2 (8.7%) T3. Preoperative clinical neck status was N0 in 10 patients (58.8%), N1 in 6 (35.3%), and N2 in one (5.9%) whilst pathological neck stage was N0 in 10 patients (58.8%), N1 in 4 (23.5%), and N2 in 3 (17.6%).
On histological examination, according to the Broders classification, the tumor was well differentiated in 8 patients, well to moderately differentiate in 6, and moderately to poorly differentiate in 3. All patients were treated by partial glossectomy and neck dissection. Five patients underwent postoperative radiation therapy. As the control group we had 8 patients with reactive fibroma of the tongue. The control group consisted of 4 males and four females, with an average age of 46.8 years, all treated by excision.
Follow up period was 5 years. All patients were followed every month for the first postoperative year. Computed tomography and ultrasonography were performed on the basis of clinical need. Ten patients (58.8%) failed treatment within the first 24 months of follow-up. Of these 7 had a recurrence of the primary disease (43.8%), 2 had neck metastasis as well as recurrence in the tongue and floor of the mouth; and one had only neck metastasis. By the end of the follow-up period, 10 patients (52.9%) had died of the disease and 7 patients (41.2%) had no evidence of disease (Table 1). In the control group, follow-up time after pathological diagnosis was 4 weeks. No further treatment was needed after excision.
All 25 specimens were successfully stained, and CD-117 positivity was observed in all specimens. In the study group, staining was detected mainly in the cytoplasm of the stromal spindle cells, but not in the tumor cells (Table 1). The CD-117 label index of 56.6%+- in the spindle cells at the interface of the invasion front of the tumor. In the control group, there was a of CD-117 label index of 28.2%+- (Table 2). This difference was significant (p<0.001). The study group was further divided according to clinical course and outcome. Group 1 consisted of the 7 patients with no evidence of disease (Ned) at the end of the five years follow-up period; Group 2 consisted of the remaining 10 patients who died of the disease due to loco-regional failure or distant metastases. Group 1 had a label index of 42.6%+- whereas group 2 had an average of 64%+-. However, this difference was not statistically significant (P=0.19).
Table 1: Results for CD-117 reactivity in 17 patients with SCC of the tongue.
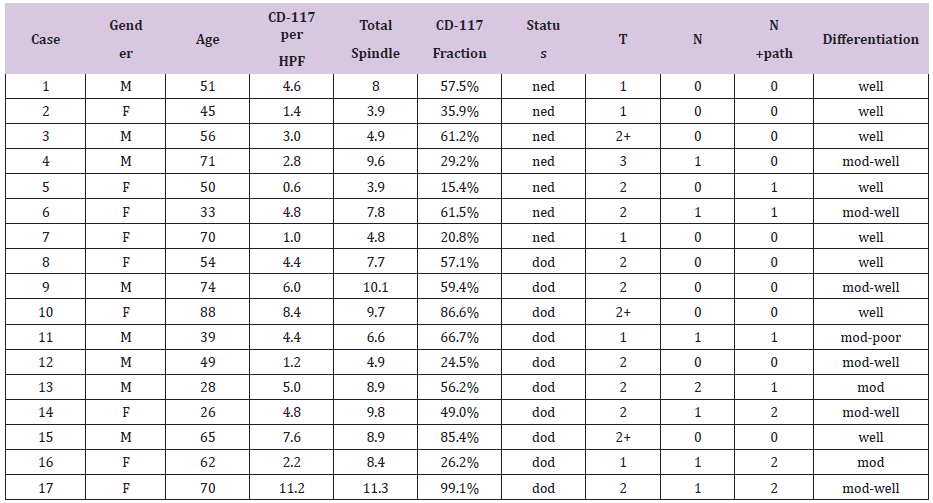
M-male, F-female, HPF- High power field, ned-no evidence of disease, dod-died of disease, n+path- pathological neck staging, dissection mod- moderate.
Table 2: Results for CD-117 reactivity in 8 patients with irritation fibroma of the tongue.
Carcinomas are not merely an uncontrolled proliferation of malignant epithelial cells, but also consist of a dense, fibrous, tumor-induced, surrounding host tissue2; [32-35] comprised of fibroblasts, macrophages, lymphocytes, polymorph nuclear cells, mast cells, and endothelial cells. In addition to the remodeled stroma, the tissue contains extracellular matrix, growth factors, regulatory molecules and remodeling enzymes, and proteases and their inhibitors the desmoplastic reaction whereby the tumor cells induce stromal remodeling involves processes of inflammation and angiogenesis, matrix production, and tissue lysis and absorption [34]. This cascade has some similarities to wound healing. Normally, the basement membrane, composed of collagen type IV, laminin, heparan sulfate, proteoglycans, and other macromolecules, is capable of preventing the passive migration of tumor cells from their tissue origin. Therefore, the degradation of the extracellular matrix, including the basement membrane component of the vessels and interstitial stroma, is a key element in cancer progression. Most of the degradation enzymes involved in the invasion of cancer cells is produced by the altered stromal tissue.
These changes are expected mostly in invasive carcinoma and not in benign tumor or carcinoma in situ. The presence of stromal changes in carcinomas otherwise considered noninvasive is of high prognostic significance [21,34,36-38]. The various structural changes induced in the stroma by epithelial malignant cell activity are consistent in various sites and histological types of carcinomas. In carcinomas of the breast, gastrointestinal tract, and uteral cervix, where fibroblasts are a normal part of the dermis and connective tissue, three major histological trends have been identified: gradual loss of fibroblasts followed by an elevation in the number of my fibroblasts positive for α-smooth muscle antigen (SMA) and the appearance of CD-117-positivefibroblasts.
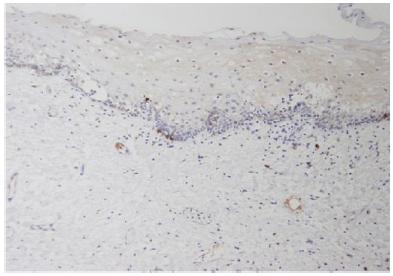
Fibroblasts play several roles in the regulation of tissue growth and repair. They are matrix-producing and antigen-presenting, and they also secrete platelet-derived growth factor (PDGF). The distribution of my fibroblasts containing α-SMA, vimentin, desmin, and common leukocyte antigen has been studied in SCC and other epithelial hyperplastic lesions of the larynx.35Well- differentiated SCC was found to be characterized by marked proliferation of my fibroblasts and desmoplasia, with only scattered lymphocytes. Poorly differentiated carcinoma showed only few my fibroblasts and a heavy lymphocytic reaction. Normally, activation of the receptor tyrosine kinase CD-117 by the kit-ligand (SCF) [15,20,39,40] mast cell growth factor [5] or steel factor, [6-8] is essential to melanocyte and germ cell development and the early stages of hematopoiesis. Uncontrolled expression of the c-kit protooncogene is characteristic of some malignancies, including myeloid leukemias, [41] where the exact mutation site on the c-kit gene has been localized, [12] in addition to melanomas, [42-44] and germ cell tumors. [45] Expression is most intense in mast cell diseases [9-13]; testicular germ cell tumors, [21,24,36,37] endometrial carcinomas, [46] papillary and follicular thyroid carcinomas, [16,47] small cell lung carcinomas, [26,48] malignant melanomas, [42-44] and ovarian epithelial carcinomas express altered levels. Adenoid cystic carcinoma of the salivary glands expresses CD-11749 in correlation to its histological grade [49,50]. Several studies have suggested that c-kit plays an autocrine role in normal or malignant epithelial tissues. [51-53] accordingly, the over expression of both c-kit and its ligand, SCF, in colon cancer cells relative to cells of normal mucosa [54]. Apparently establishes an autocrine c-kit-mediated loop, [19] improving the invasive capability of the tumor cells and protecting them from programmed cell death. [55] A similar co-expression was also noted in some breast [19,51] and gynecological tumors [52]. Furthermore, expression of the potential autocrine SCF/ c-kit axis in gastrointestinal carcinoma cell lines was inhibited by transforming growth factor (TGF)-beta [56] and interleukin [57]. Others suggested that mutations in the c-kit genes were involved in the pathogenesis of several neoplasms (Figure 1).
Figure 1: Normal mucosa (original magnificationX40) showing the majority of fibroblasts negative for c-kit. Inset (at magnification X200) exhibiting rare positive cells.
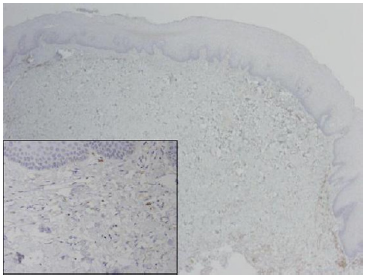
This finding was attributed, in some types of carcinoma, to genetic alteration of the c-kit juxtamembrane domain (exon 11) and tyrosine kinase domain (exon 17) during the process of malignant transformation. [49,58] the expression of two types of aberrant c-kit transcript mRNAs has been observed in various cancer cell lines. [59] In SCC of the upper aerodigestive tract, CD-117-positive fibroblasts have been reported in the stroma, along with a gradual loss of fibroblasts and elevation in SMA-positive myofibroblasts. [32] However, the authors themselves noted that chronic inflammation might also be accompanied by a focal loss of CD34+ with no effect on CD-117 levels. Another study indicated that in head and neck SCC, immunosuppressive CD34+ progenitor cell levels increased in the stroma; this elevation was also demonstrated in peripheral blood [60]. In cultures, granulocyte-macrophage colony-stimulating factor (GM-CSF) induced the proliferation and differentiation of CD34+ cells into dendritic cells. The interaction between CD34+, CD-117+, my fibroblasts, and mast cells is complicated and not entirely clear. The presence of CD-117+ my fibroblasts in tumorassociated but not in tumor-free stroma indicates that SCF or PDGF or both might play a role in their proliferation. Additionally, GMCSF is closely related to macrophage colony-stimulating factor (M-CSF), which is constitutively expressed in fibroblasts. [61] Given that GM-CSF apparently down regulates CD-117 expression in mast cells and in stromal my fibroblasts, [62] a reduction in fibroblasts would be expected to lead to up regulation of CD-117 in stromal my fibroblasts and in mast cells. Since fibroblasts serve as antigenpresenting cells, their reduction or elimination from the stroma makes it possible for invasive carcinoma cells to escape immune surveillance and perform infiltration and distant metastatic spread. The results of the present study suggest that higher labeling index of positive c- kit fibroblasts in an oral SCC- supporting stroma, may be serve as a diagnostic marker for more aggressive and infiltrative biological behavior of the tumor. Furthermore, a recently FDA approved chemotherapy agent, Imatinib mesylate, a competitive inhibitor of tyrosine kinases, including cd-117and the plateletderived growth factor receptors (PDGF-R), binds to the ATP-binding site of the target kinase and prevents the transfer of phosphate from ATP to the tyrosine residues of various substrates (Figure 2).
Figure 2:SCC tumor front (original magnificationX100) exhibiting multiple fibroblasts positive for c-kit. Inset showing several positive spindle cells between tumor islands.
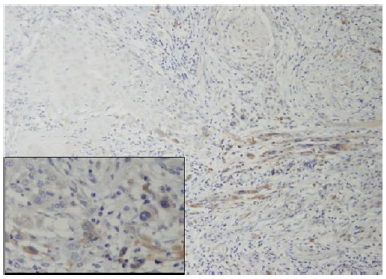
Up to date, this agent is approved for use only in chronic myeloid leukemia, Philadelphia chromosome-positive acute lymphoblastic leukemia expressing the BCR-ABL fusion protein and gastrointestinal stromal tumors (GIST). At oral doses of 200- 600 mg, a clinical response, frequently complete, is associated with limited toxicity. In other types of CD-117 associated tumors (Figure 3), such as small-cell lung carcinoma, melanoma, seminoma, some sarcomas, and adenoid cystic carcinomas and clinical trials to evaluate the role of Imatinib mesylate in the treatment of such cancers are currently ongoing [62]. The aforementioned finings, may support the rational, that more aggressive oral SCC with evidence of stromal induction via the tyrosin-kinase cd-117 axis, is expected to respond to inhibition of this mechanism by a selective agent such as Imatinib mesylate.
Figure 3:SCC tumor front (original magnificationX100) exhibiting multiple fibroblasts positive for c-kit. Inset showing several positive spindle cells between tumor islands.