Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
M Wolf1*, B Streit2, M Dokládal3 and M Schultz4
Received: August 17, 2017; Published: August 28, 2017
Corresponding author: M Wolf, Former, Department of Mecklenburg, Vorpommern, Wiligrad, Germany
DOI: 10.26717/BJSTR.2017.01.000304
For age determination of cremated bones of variable stages of preservation the method of Kerley and Ubelaker [1] was chosen. As a new criterion to determine individual age the lamina fundamental is internal of the compact bone was used [2]. Examinations of the intact osteons of individuals from Roman graveyards show that after cremation at temperatures between 450 and 650°C age determination is possible only by counting central haversian canals. At this temperature level, a qualitative age determination is limited. The examination of modern postmortem bones indicates that the four criteria defined by Kerley [3] are necessary to determine the physiological age of cremated human bone specimens with different burning degrees more precisely and that the lamina fundamental is internal should be included as an additional criterion.
Keywords: Cremated Bones; Chronological Age; Field Size; Haversian and Non-Haversian Canals; Lamina Fundamentalis Interna and Externa; Macroscopic Age; Microscopic; Histomorphometric Age; Osteon Fragments; Physiologial Age
The physiological age of a cremated human being can only be elucidated from the skeletal maturity state of the individual, and represents not more than a rough estimation. According to the classification by Martin [4], bones of adult cremated individuals are classified at age intervals of twenty years or with a minimum age, if there are no other indication factors. The age diagnosis of children has its starting point in the dentition and the mineral content of the milk teeth. Furthermore the change to permanent dentition before the eruption of the second permanent molar at the age of twelve is a reliable indicator of age. It has to be taken into account that the growth of the epiphyses is regulated by hormones and differs between males and females. On average the epiphyses of females fuse at an age between 18 and 20, and of males between 20 and 22. As far as histomorphometric age determination is concerned, various methods have been published [4-8]. These methods are based either on the determination of osteons and osteon fragments per unit area by counting the number of osteons. However, difficulties do occur with cremated specimens if the applicability of a method is dependent on a very precise distinction of single structural elements, such as intact or fragmented osteons. Especially the methods of Ahlquist and Damsten and Drusini deal with these difficulties. The objective of the present study was to gain a more precise determination of age by using a histomorphometric method as an additional criterion. Only Kerley`s method employes two more criteria, the non-haversian canals and the lamina fundamentalis externa, therefore this method was used in the present study.
A histomorphometric age determination was made for 235 out of 245 individuals from two Roman graveyards, dated between 30 and 260 A.D., near the village of Rheinzabern, 25 km southwest of Speyer, Rheinland-Pfalz.
Histomorphometric data were collected from 640 calcified thin sections of the midshaft femora and the midshaft tibiae of the archaeological population. The quality of microscopic research relies not only on experience but also on a sufficiently good technique for the production of thin sections of dry bone samples [9-11]. Because of the fragmented nature of the analyzed remains, each femoral and tibia sample required embedding in plastic (BiodurTM) before sectioning, following the method of Schultz and Brandt (in press) and Schultz and Drommer [12]. The method used for embedding the cylinders was designed to produce a block that could be mounted easily in a wafering-saw chuck so that the surface of the femur piece (encased within the block) was orientated at right angles to the wafering blade. (i.e. wafers were parallel to the z-axis of the diaphysis).
The embedded bone was placed under vacuum for 7-10 hours to remove air bubbles and to adequately infiltrate the bone specimen. After the BiodurTM had hardened (2-3 weeks at room temperature), the metal mantle was peeled away from the blocks with pliers. The trimmed block of approximately 10 mm thickness was then put under sub-atmospheric pressure on a metal fastening, which run vertically to the wafering blade. Two cross-sections, each approximately 5 mm thick, were removed from each block at the midshaft mark (which was visible through the clear embedding medium) on a diamond wafering saw. Each half was ground and polished using P 320 and P 1200 KarborundumTM grit paper and a polishing paste from a polishing stone. The polishing paste was rubbed into a leather block used for polishing. The cleaned and dry cross-sections were mounted onto 28 mm x 48 mm and 40 mm x 60 mm labeled microscope slides using BiodurTM. The sections were then prepared for histomorphometric analysis by reducing them, still secured to the slides, to a thickness of (120 m) on a precision grinding machine (MPS 2-120, G & N TM) with a diamond disc (400 x 1.0 x 20 mm, Richard HahnTM). For final grinding P 320 and P 1200 grit paper and the polish stone were used again. To complete the histomorphometric preparation, glass cover slips were applied to the thin sections.
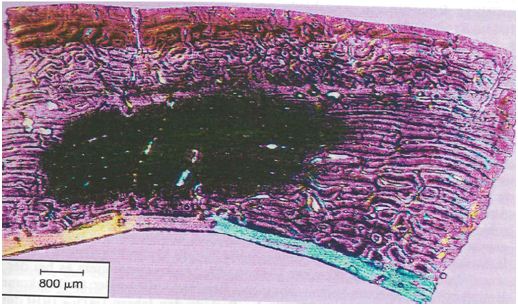
Figure 1: Undecalcified cross section of the femur (70mm cross section) covered by a 10 x 10 grid (Zeiss TM) 100 x field size under polarized light using quartz in order to enhance contrast (Kerley’s method, adult, unsexed, tendency female, 22 years according to intact osteons (41)).
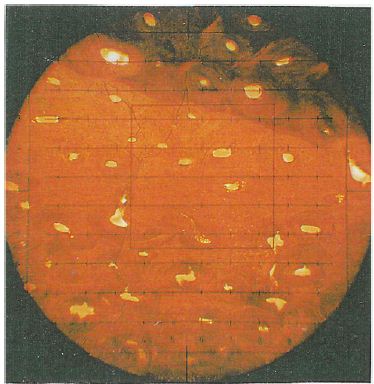
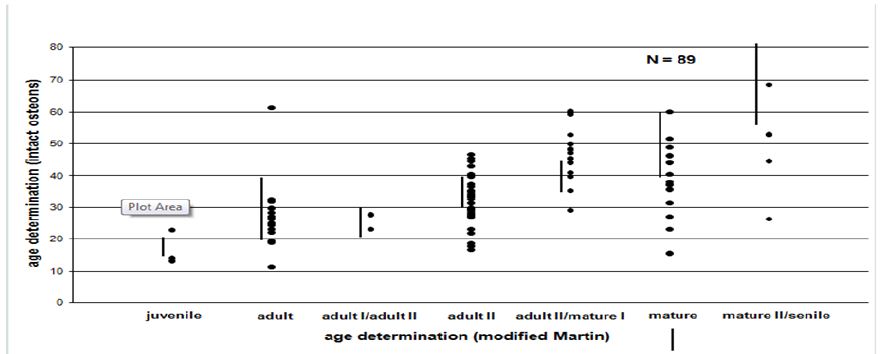
Figure 2: Comparison between macroscopic and histomorphometric age.
The microscopic changes taking place during aging of human cortical bone were measured by counting the number of intact osteons, osteon fragments and non-haversian canals, and estimating the percentage of lamellar bone in four selected 100 x power fields in the outer third of the cortex in the mid shaft of femur, tibia and fibula. Using Kerley’s method of age determination the variable field diameters should be taken into account and should by adjusted to a field diameter of 1.62 mm. The area ( x r2) of the 100 x power field must be calculated using a stage micrometer and the field size divided into 2.06 mm2 (area of the field diameter of 1.62 mm) to determine the relationship between the original field size and the field size of the individual microscope being used. All findings on osteons, fragments or non-haversian canals should then be multiplied by this factor. Structures like the haversian canals of the intact osteons with more than half of their area outside of the object field were not included. In order to facilitate the examination a 10 x 10 grid defined in (Figure 1) was used. The mortality age is calculated using the regression equations presented in the study [1]. Only those structures were counted which were visible for at least half of their total extension. Additionally, the influence of shrinking was examined. Twelve modern postmortem proximal human femora pieces (from six individuals) from the pathology department of the Masaryk University in Brno (Czech Republic) were examined. The bones were prepared following the method of Dokládal [13]. As a new criterion for the age diagnosis the lamina fundamental is internal was used (Figure 2).
For adult individuals the fragmentary and non-representative cremated bones allowed for an age determination using macroscopic criteria at simple ten to twenty-year intervals, by counting intact osteons and using the regression equations of Kerley & Ubelaker [1]. The histomorphometric age is determined as an absolute age. The explanation for the great variation between histomorphometric age and macroscopic age lies in the use of qualitative and quantitative parameters respectively.
In half of the cases the histomorphometric age determination lies outside the defined area of the macroscopic age. Up to the mature age class the means of the histomorphometric (intact osteons) and the macroscopic age determination (Figure 3) correspond to each other more or less. In nearly 75% of the cases beyond the mature age class the histomorphometric determined age is lower than the macroscopic age. Before the age classes mature II/senile the average histomorphometric age and the average macroscopic age show some correspondence to each other. According to the macroscopic age assessment more than the half of the population can only be determined with their minimum age being “juvenile or older” or “at least adult”, if there are no other indications (Figure 4). According to the intact osteons the modern postmortem thin sections of the mid shaft femora indicate a remarkably high divergence with regard to the absolute age, except for one individual (Figure 5). It is remarkable that the analysis of quantitative parameters like the fragmented osteons and the lamina fundamental is internal and external evidently leads to an older age determination than the examination of intact osteons. This result cannot be corroborated by the findings of the archaeological series from Rheinzabern (Figure 6). A better correspondence was achieved using the four criteria by Kerley [3] and the lamina fundamentalis interna.
Figure 3: Average histomorphometric age in comparison to macroscopic age.
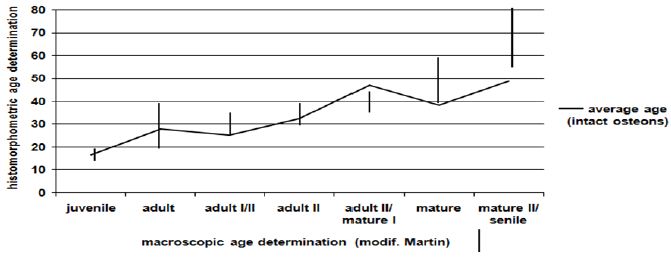
Figure 4: Minimum macroscopic age determination.
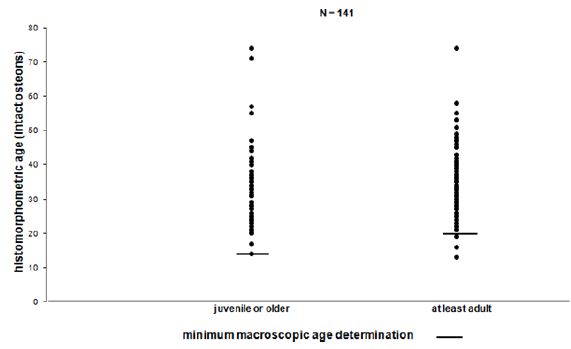
Figure 5: Minimum macroscopic age determination.
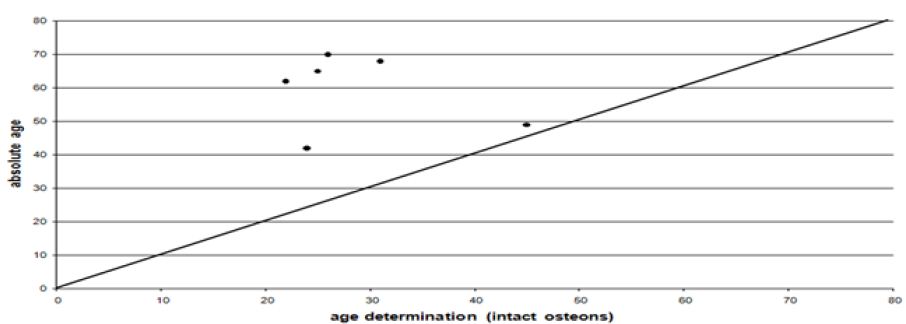
Figure 6: Correlation between the quantitative histomorphometric age and the chronological age.
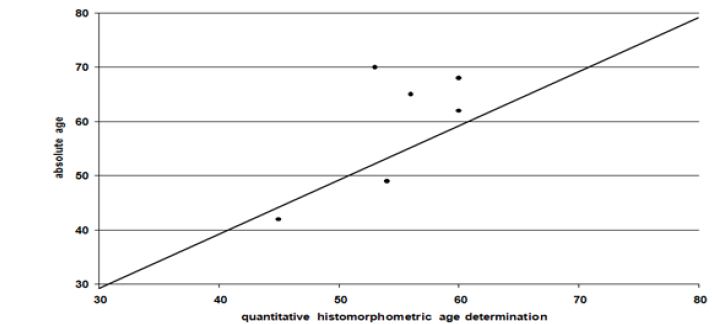
In spite of some variation there is a good correlation between the qualitative age determination by M. Schultz and the chronological age (Figure 7). Using linear regression, the age of the lamina fundamentalis interna was estimated from the intact osteons. To calculate the extent to which the lamina fundamentalis interna is included the object field had to be changed. Evidence is limited because the age diagnosis of the inner lamella is based on the estimated age whereas the chronological age is unknown (Figure 8). Obviously there is only a weak correspondence between age assessment according to the intact osteons and the lamina fundamental is internal.
Figure 7: Qualitative histomorphometric age determination and chronological age.
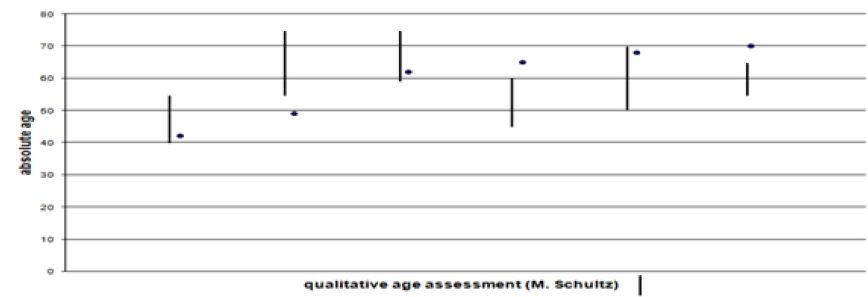
Figure 8: Age assessment according to intact osteons (x-axis) and to percentage of lamina fundamentalis interna (y-axis).
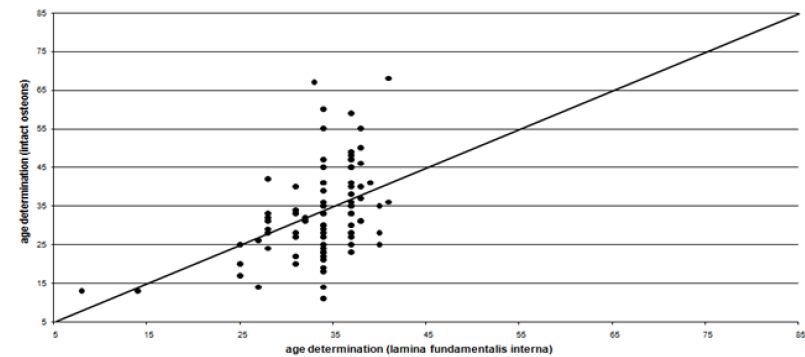
For adults the fragmentary and non-representative cremated bones provided an age determination by macroscopical criteria simply at ten to twenty-year intervals. The histomorphometric age determination is an attempt to assess age more precisely. This explains the great variation inside the macroscopic age groups (Figure 2). If there are not enough criteria for a macroscopical diagnosis, age can only be determined in groups of “juvenile or older” or “at least adult”. Therefore, using the macroscopic method more than half of the population can only be assessed in terms of these two groups (Figure 4). Considering the intact osteons the modern postmortem thin sections of the midshaft femora indicate a great divergence with regard to the chronological age, except for one individual (Figure 5). Therefore, it is remarkable that ages indicated by quantitative parameters like the fragmented osteons and the percentages of the lamina fundamentalis interna and externa are evidently older and thus more reliable than those based on the intact osteons. This result is contradicted by analysis of the archaeological series from the Roman graveyard of Rheinzabern. A better agreement was achieved using the four criteria defined by Kerley [3] and the lamina fundamentalis interna (Figure 6). Large reabsorption lacunae are responsible for the reduction of intact osteons. They reduce the age-related osteon numbers of healthy bones. This observation stands in opposition to the statement by Kerley [3] that in general the ages of normally looking bones even from individuals with skeletal pathologies could be estimated with the same reliability as bone from healthy individuals. Consequently, using intact osteons provides a younger age. Apart from one individual, among the modern bones evident traces of osteoporosis were found spreading from the linea aspera femoris, the histological structure of which is less suitable for age determination [5,7,14,15- 18]. Therefore, a general disadvantage of Kerley`s method lies in the fact that one of the object fields is situated in the area of the linea aspera [18,19]. Although there is some deviation, the qualitative age determination by M. Schultz shows a relatively good correspondence to the chronological age (Figure 7).
Figure 9: Undecalcified cross section of the femur (90m cross-section) 25 x field size in a apparent light, organic carbon (450°C), the osteons are only identified through the central haversian channel (modern male individual, 70 years (intact osteons)).
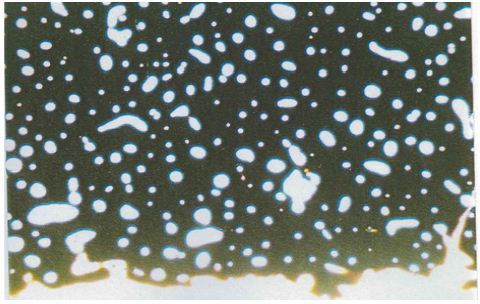
Figure 10: Undecalcified cross section of the femur (90mm cross-section) 25 x field size in a apparent light, residual organic carbon in the inner compact bone layer of the os femoris modern female individual, 68 years (intact osteons).
Specially for individuals with metabolic bone diseases the comparison of quantitative, histomorphometric methods with a qualitative method which is based on evaluating the relative amounts, size and shape of the different types of bone microstructures revealed the qualitative method to be preferable [18,20]. Hummel and Schutkowski emphasized that in their experience a qualitative age determination produces better results for unburnt bone than for cremated bone. One reason is that an age determination of bone which has been exposed to burning temperatures between 450 and 650°C is only practicable counting central haversian canals [18], so that at these temperature levels qualitative age determination is limited (Figure 9). The use of polarized light does not lead to considerable improvement, since the bone mineral becomes isotropic during the sintering process [20- 22]. For cremated human remains different burning degrees of the bones are the usual case (Figure 10). After a burning temperature between 750 and 850°C the inorganic bone structures are in a good condition for a qualitative or quantitative age assessment.
Figure 11: Undecalcified cross section of the femur (50m cross-section) 25 x field size in polarized light using quartz. The lamina fundamentalis externa has lifted and formed “blisters” the walls of which have burst.
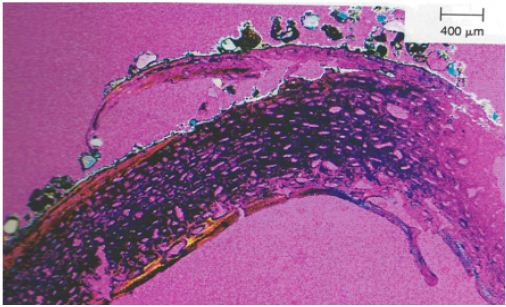
Another problem lies in the different shrinking factors. Apart from a few examples, burning temperature was never below cremation degree III (550°C, classification according to Wahl [23]). In contrast to unburnt bone the examination of burnt femora pieces of the same individual indicates a shrinking factor of approximately 25% across the section. It could be shown that the type of structural element does not influence the amount of shrinkage, i.e. the shrinkage is linear [20,24,25]. The problem is that this shrinking rate indicates an age which is 9 to 10 years younger than the actual age! Heussner [24] never found any significant shrinking factor, therefore she neglected this parameter. Our shrinking rate is in accordance with the examinations by Hummel and Schutkowski and several other groups of investigators. It is necessary to quantify the shrinking factor according to different burning degrees on the basis of a modern population, the mortality age of which is known. The cremation of female and infantile burials is more varied compared to the male cremations. This is due to the higher collagen content and the lower content of minerals in the bones [21,22,25,26]. Several cremation degrees of the bones are the general case for archaeological urn fields (Figure 10 & 11).
The archaeological bone specimens were made available by Dr H Bernhard of the Archaeological Department in Speyer (Rheinland-Pfalz, Germany). We would like to thank the team of Prof Dr Dr M Schultz, Mrs Hettwer-Steeger and Mr Michael Brandt from the Department of Anatomy of the Georg-August-University (Germany), for embedding and trimming the bone specimens.


