Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Sahar Emami*
Received: August 24, 2017; Published: September 08, 2017
Corresponding author: Sahar Emami, Department of Veterinary, Shushtar Branch, Islamic Azad University, Shushtar, Khuzestan, Iran
DOI: 10.26717/BJSTR.2017.01.000338
Background: Tuberculosis (TB) is a major public-health problem which principally affects the lungs, but may present a variety of rheumatic manifestations, simulating rheumatologic disorders such as rheumatoid arthritis.
Methods: The main aim of this research was examined the anti- ccp level in 40 patient with active tuberculosis and 40 normal person’s serum by ELISA.
Results: Mean levels of anti-CCP were significantly increased (p<0.01) in patients with TB compared with controls: 29.8 (17.7) vs. 10.8(7.9) U/ml, respectively.
Conclusion: Finally, a high percent of patient with active TB show an increased level of anti–ccp that could make difficulties to detecting TB Arthritis.
Keywords: Tuberculosis; Cyclic Citrullinated peptide antibody
Abbreviations: TB: Tuberculosis; RA: Rheumatoid Arthritis; RFs: Rheumatoid Factors; PAD: Peptidyl Arginine Deaminase; ANA: Antinuclear Antibody; Anti-CCP: Anti-cyclic Citrullinated peptide
Tuberculosis (TB) is a major cause of human mortality and a worldwide public health problem .TB is a chronic, communicable disease caused by M. tuberculosis made distinctive by a destructive necrotizing granulomatous tissue response to the organisms. Mycobacterium tuberculosiscauses two million deaths every year and every year 8 million of new cases of TB are diagnosed worldwide [1,2]. Both pulmonary TB and also cases of extra pulmonary disease are increasing [3]. About 10% of extra pulmonary TB affects the bones and joints [4]. Tuberculosis of bones and joints often presents as graduallyworsening arthritis. Systemic and pulmonary symptoms are frequentlyabsent, and the differential diagnosis must include other possiblecauses of septic osteoarticular disease, inflammatory arthritis.
Osteoarticular tuberculosis rarely involves more than one joint and this may help to differentiate it from other polyinflammatory diseases. Also, the radio graphic changes of tuberculosis are slow to develop compared with those of pyogenic infections, and a reduction in the joint space is often a late occurrence [5]. Clinical confusion occurs especially when tuberculosis superinfects joints previously involved by inflammatory arthritis [6]. TB arthritis most commonly manifests as a mono arthritis of the weight bearing joints [7], but oligoarticular or poly articular presentation is not rare and these findings may mimics inflammatory diseases such as the spondyloarthropathies [8] or Rheumatoid Arthritis (RA), or both [9]. Also, the serum of patients with TB may contain Rheumatoid Factors (RFs) in up to 40% of cases [10]. Patients with TB even those without evidence of a direct musculoskeletal/local involvement, may present with a variety of rheumatic symptoms and signs including arthralgia and arthritis.
The presence of rheumatic manifestations can lead to challenges in making or excluding a diagnosis of rheumatoid arthritis (RA) in patients infected with TB. For distinguishing between true RA and TB rheumatic symptoms, the presence of RF is of little help and can be confusing or misleading. Anti-cyclic Citrullinated peptide (Anti-CCP) was detected in the serum samples of RA patientswhich shows high diagnostic accuracy. Citrulline is an unusual amino acid created by de-imitation of arginine residues in several proteins by the action of peptidyl arginine deaminase (PAD). Theantibodies are detected by ELISA, where a synthetic cyclic citrullinated peptide (CCP) is used as substrate [11].
50 serum samples of patients with active lung tuberculosis (29 men and 11 woman) diagnosed according to clinical symptoms, radiologic studies, and laboratory confirmation including direct smear of sputum and sputum cultures for Mycobacterium tuberculosis and consecutively recruited from the department of tuberculosis and pulmonary disease in 5th Aboozar Hospital, in Ahvaz (Iran), an endemic area of tuberculosis Also 50 sex and age matched healthy subjects are studies for serum anti-ccp levels. Among the TB patients, the variables recorded were age, gender, time from diagnosis, duration of symptoms, fever, hemoptysis, cough, myalgia, and arthralgia and serum titer of anti-ccp. Anti-CCP antibodies were tested by commercial ELISA (Aeskulisa; Aesku. Diagnostics, Germany).
Each of the tests was performed and evaluated by operators who were blinded to the patients’ clinical data, and radiological and microbiological results. The assay was performed according to the manufacturer’s instructions. To determine the precision of the assay, the variability (intra and inter-assay) was assessed by examining its reproducibility on three serum samples selected to represent a range over the standard curve. Analytical sensitivity was 1.0 U/ml. Intra-and inter assay CVs were 0.4-8.9% and 1.0- 8.2%, respectively. All moral standards in this study were compiled and the project was approved by the University’s ethics committee.
Analysis was performed using the SPSS version 13, Student’s t test and a χ2 test to compare antibody titersor positivity rate, respectively, between patients with TB andcontrols. Pearson correlation coefficients were used to studythe relationship between clinical measures and the level of anti-CCP. A value of p<0.01 was considered significant.
Analysis was performed using the SPSS version 13, Student’s t test and a χ2 test to compare antibody titersor positivity rate, respectively, between patients with TB andcontrols. Pearson correlation coefficients were used to studythe relationship between clinical measures and the level of anti-CCP. A value of p<0.01 was considered significant.
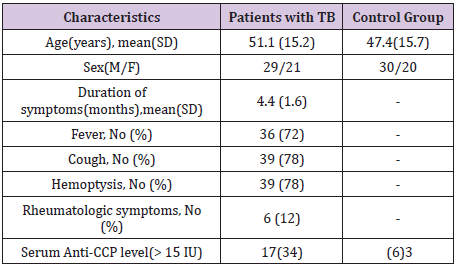
Table 1: Demographic, clinical characteristics and anti-CCP levels of patients with TB and healthy controls.
The patients (mean and SD 51.1+/-15.2 years; range, 19-81 years) and healthy subjects (mean and SD 47.4+/-15.7 years; range 23-85 years) ages were determined. The mean (SD) duration of TB related symptoms was 4.4 (1.7) months, 72% had fever, 78% hadcough and 78%had hemoptysis. Rheumatic symptoms were relatively rare: arthralgia (2%), myalgia (4%), and eye and mouth dryness (1% and 3%, respectively). Mean (SD) levels of anti-CCP were significantly increased in patients with TB compared with controls: 29.8 (17.7) U/ml v 10.8 (7.9) U/ml (p < 0.001). Serum levels >15 U/ml were found in 17 (34%) patients compared with 2 (6%) controls (p = 0.002). Risk of pulmonary TB in subjects with anti-CCP serum level higher than 15 U/ml is almost 12.5 fold persons lower than the cut-off point (odds ratio=12.4, CI 95%: 2.6- 114.9). No relationship was found betweenthe titer of anti-CCP and fever and any rheumatic symptom (Table 1).
We sought to determine if anti-CCP was also associated with active pulmonary tuberculosis. The data presented here clearly indicate that anti-CCP can be found in the sera of unselected patients with TB. We found that 17 (34%) patients with TB havepositive levels of anti-CCP. Although the presence of anti-CCPcorrelated with fever, it was not associated with symptoms and signs of arthritis. Elkaya et al. [12] showed significantly increased anti – ccp levels in tuberculosis patients in comparison with controls. In this study, 32 percent of patients in comparison with 2.6 percent of controls show serum levels above the upper normal limits. They found correlation between increased serum anti – ccp and a history of prolonged of fever, as we noted in the present study. As we revealed, no correlation was found between anti – ccp level and rheumatic symptoms in Elkayam study [12].
Patients with TB may have a variety of rheumatic symptoms andsigns, including arthralgia and arthritis and TB may imitate inflammatory rheumatic diseases, such as RA [13]. Also the radiological abnormalities observed in tuberculosis arthritis may simulate RA [14]. Antibodies to CCP are present in the sera of a majority of patients with RA, only rarely in the sera of patients with other diagnoses. TB of the bones and joints, which constitutes a smaller part of extra pulmonary tuberculosis, is relatively rare in documented cases and can yield variable clinical manifestations. Many authors describe numerous cases of tuberculosis arthritis in patients presenting with mono arthritis [15-20]. Tuberculosis must always be considered in the differential diagnosis of chronic monoarthritis if devastating squeal is to be avoided.
In recent years, various antibodies have been described in rheumatoid arthritis, and their clinical significance has been discussed. In particular, autoantibodies to citrulinated proteins such as fill grin and its circular form (cyclic citrulinate peptide: anticcp) are the most remarkable because of the reasonable sensitivity and high specificity in RA patients [21]. They are presenting most patients with RA and have been found to have a specificity of >90% [22]. Although anti-CCP may present in the serum of patients with psoriatic arthritis and have questioned its specificity [23]. The presence of anti-CCP in a few infectious diseases, ashepatitic C has been studied. Although, rheumatic factor (RF) is present in the great majority of patients with hepatitis C arthritis and in proportionof patients with other sub acute infections, particularly bacterial endocarditis, anti-CCP is negative in patients with HCV andmeasurement of anti-CCP may help in diagnosing RA in patients with chronic HCV infection [24].
Patients with active tuberculosis may have increased titers of various autoantibodies and serologic markers, such as rheumatic factor (RF), antinuclear antibody (ANA), anti cardiolipins, anti neutrophilic cytoplasmic antibody, anti-ds DNA, anti-Sm, anti- RNP and anti-Ro [25-27]. Genetic factors such as HLA and agene polymorphism of the citrullinating enzyme, peptidyl arginine deiminase, (that might express more stable mRNA and cause overcitrullination of proteins) might be associated with the breakage of self-tolerance and induction of autoimmunity against Citrullinated proteins [20-28]. It is not clear whetherthe false positive anti-CCP reactivity seen in patients with TB is directed against citrullinated or non-citrullinated epitopesin the substrate for the CCP test [12].
A relatively high percentage of patients with active pulmonary tuberculosis show increased titers of anti-ccp which could impede diagnosis of articular TB. Therefore, it is recommended not to limit the diagnosis of arthritis types to anti-ccp test, and perform other assessments in order to differentiate articular TB.


