Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
TS Sampath Kumar1*, V Yogeshwar Chakrapani1,2, Pooja Vardhini Natesan1, Deepa K Raj2 and TV Kumary2
Received: September 09, 2017; Published: September 21, 2017
Corresponding author: TS Sampath Kumar, Medical Materials Laboratory, Department of Metallurgical and Materials Engineering, Indian Institute of Technology Madras, Chennai-600036, India
DOI: 10.26717/BJSTR.2017.01.000379
Controlling the biodegradation of scaffolds to tune with that of the growing tissue is crucial for tissue regeneration. Although molecular weight of a polymer is known to influence its degradation, such studies have not been reported for electrospun Polycaprolactone (PCL) based composite scaffolds. In the present study, two low molecular weight PCL, 10 and 45 kDa PCL (10PCL and 45PCL) have been analyzed for their electrospun ability and biodegradation in comparison with 80 kDa PCL (80PCL) in forming pure polymeric and hydroxyapatite composite nanofibrous mats. The 45PCL and 80PCL were electro spin able but the 10PCL resulted in electro spraying of particles. However, 45PCL needed a higher concentration (20 wt.%) of polymer than the 80PCL (8 wt.%) in order to electrospin bead free uniform fibres. The fibres obtained were in the diameter range of 310±50 nm and 400±100 nm for the 45PCL and 45PCL/HA composite mats respectively. The Hydroxyapatite (HA) incorporation in the composites was confirmed through X-ray diffraction and spectroscopic methods. The 45PCL/HA composite degrades at a relatively faster rate than the 80PCL/HA composite. The electrospun mats were also found to be non cytotoxic (Live/Dead assay), commendable cellular metabolic activity (MTT assay) and good proliferation of osteoblast like cells (HOS) as evidenced through fluorescent and scanning electron microscopy. This work demonstrated the feasibility of fabricating PCL based electrospun composite scaffolds having controlled degradation rates.
Keywords: Electrospinning; Low molecular weight; Controlled degradation; Bone tissue engineering
Abbreviations: PCL: Poly Capro Lactone; ECM: Extra Cellular Matrix; HA: Hydroxy Apatite; MW: Molecular Weight; HOS: Human Osteo Sarcoma; HV: High voltage; ATR : Attenuated Total Reflection
Electro spinning is commonly utilized for forming nanofibrous scaffolds, to mimic the extra cellular matrix (ECM), for tissue engineering applications. Polycaprolactone (PCL) is often favored for Electrospinning of tissue engineering scaffolds. One of the reasons for its widespread usage is PCL’s ability to degrade in physiological conditions, by hydrolysis of its ester linkages [1], into intermediates such as 6-hydroxylcaproic acid and acetyl coenzyme A, which are eliminated from the body through the citric acid cycle [2]. However, PCL degrades very slowly due to the five hydrophobic CH2 moieties in its repeating units (Figure 1) [3].
This has been the reason for denying the ‘ideal polymer’ status for PCL in orthopedic applications in spite of its promising results [4]. Methods to improve its degradation include blending with hydrophobic polymers [5], adding ceramic fillers and plasma treatment [6]. Hydroxyapatite (HA) is the most commonly studied ceramic filler used for fabrication of PCL based composites for bone tissue engineering due to its similarity to the natural bone mineral through which properties such as bioactivity, osteoconductivity and osteoinductivity can be imparted to the electrospun scaffolds. HA can also provide a buffering effect for the acidic by-products of polymer degradation [7].
It is well known that molecular weight (MW) influences the kinetics of degradation. Higher MW has larger chain lengths, thereby necessitating more number of ester bonds to be cleaved for subsequent degradation [8]. Studies on PCL implants have observed a reduction in the MW of the starting material, only after which fragmentation of the low MW PCL occurs [9,10]. Generally PCL with a MW of 80 kDa or higher is used for Electrospinning and studies on low MW PCL (LMWPCL) are very few and not comprehensive in nature [11, 12]. This may be due to the difficulty in forming an electrospinnable solution using the LMWPCL polymer. In Electrospinning, the polymer chain entanglements serve to stabilize the jet and form continuous fibres [13].
Below a critical concentration or number of chain entanglements (entanglement density), brittle fibres, heavily beaded fibres or particles are formed [14]. In a polymer solution both concentration (and volume fraction) (C) and MW affect the entanglement density [14]. In the current study, two LMWPCLs, namely 10 and 45 kDa (10PCL and 45PCL) have been analyzed for their electrospinnability by comparing it with the typically electrospun 80 kDa PCL (80PCL). Additionally composite scaffolds have been electrospun by incorporating HA particles in the electrospinnable PCL solutions. The electrospun scaffolds were characterized for their physicochemical properties and their degradation behaviours were compared. The cytotoxicity and cytocompatibility of the scaffolds were analyzed through Live/Dead and MTT assay while the growth and proliferation of human osteosarcoma (HOS) cells on the scaffolds were analyzed using fluorescent microscopy and scanning electron microscopy.
PCL (10, 45 and 80 kDa), lipase enzyme and cell culture reagents were purchased from Sigma Aldrich, USA. Unless otherwise mentioned all chemicals were purchased from MERCK, India. HA was synthesized by a microwave assisted method locally as reported earlier [15].
An 8 wt. % solution of 80PCL was prepared using chloroform and methanol (3:1). Various concentrations of 45PCL (10-20 wt. %) and 10PCL (15-30 wt. %) were prepared using the same solvent system and tested for electrospinnability. To the final electrospinnable concentrations, known amounts of microwave synthesized nano HA were added, so as to obtain different PCL/HA ratios (95/5, 92/8, 90/10) and stirred for 48 h to form the composite solutions. Electrospinning was carried out using an in house developed Electrospinning setup comprising a high voltage (HV) unit (Model 2-A, Zeonics Systech, India), syringe pump (NE1000, New Era Pump Systems Inc., USA) and a grounded metallic collector. Solutions were loaded into a 10 ml syringe fitted with a blunted 22 gauge needle. The anode of the HV unit was connected to the needle tip. The metallic collector was covered with an aluminum foil on which the electrospun mats were collected. The ratio resulting in best electrospun composite mats was used for further studies. For comparison purposes a similar ratio was prepared using 80PCL and HA. Microscope cover slips, cleaned with ethanol, were placed at random positions over the collector for collecting samples for cell culture studies.
The electrospun mats were sputter coated (Gatan, USA) with gold and analyzed using a SEM (FEI- Quanta 400, Netherlands) at an accelerating voltage of 10kV. The images were analyzed using image processing software, Digimizer®. Fibre diameter was measured randomly at 50 places in each image and averaged. Additionally the elemental composition of the samples were also analyzed using EDS attached to the SEM.
The electrospun mats and HA were analyzed for their crystallinity and sample composition in an X-ray diffractometer (D8 DISCOVER, Bruker, USA) with Cu Kα radiation (λ = 1.54Å) at a scanning speed of 0.1°/ step and scanning rate of 1 step per second. The peaks of HA were indexed according to JCPDS file no. 9-432.
The electrospun mats were analyzed for their functional groups within the spectral range of 4000-510 cm-1 at a resolution of 4 cm-1 using a FT-IR spectroscopy (Spectrum Two, Perkin- Elmer, USA) fitted with an attenuated total reflection (ATR) attachment.
The hydrophilic natures of the electrospun mats were assessed by evaluating the contact angle made with a drop of double distilled water on them using a contact angle measuring instrument (Easy Drop, KRUSS, and Germany). Averages of 5 measurements were taken from triplicates of each sample.
The enzymatic degradation of the electrospun mats was carried out in a 0.1M phosphate buffer solution (PBS) with lipase enzyme at a concentration of 1 mg/ml and 0.02 % sodium azide as a bacteriostatic agent for 6 weeks. 2×2 cm2 samples were cut from the mats, weighed (WI) and incubated at 37°C in 20ml of the solution. Samples were removed every week from the solution, rinsed with deionized water and gently pressed between two filter papers to remove excess water before being dried in a vacuum desiccator and weighed to obtain the dry weight (WD). The weight loss was calculated using the formula Weight loss (%) = {(WI – WD) / WI} × 100 the change in pH of the PBS solution as the degradation proceeded was also assessed every week using a pH meter (Eutech pH 700, Eutech Instruments, Singapore)
The cytotoxicity and cytocompatibility of electrospun mat was evaluated by cell adhesion studies and MTT assay respectively. Briefly human osteosarcoma (HOS) cells were sub cultured and seeded to electrospun mat at a density of 1×104 cells/cm2 and incubated for 48h. After incubation the cells seeded mat was evaluated by live dead staining, actin-phalloidin staining, SEM and MTT assay. Test materials were sterilized with 70 % ethanol for 10 min and subsequently washed thrice with culture medium containing serum.
In order to evaluate the cytotoxic response of materials, the viability of the cells on the test samples, was assessed using Live/ Dead assay. Briefly, cells at a concentration of 1×104 cells/cm2 were seeded on the test materials and glass cover slips (control), and incubated for 48 h. Subsequently the cells were stained with fluorescein diacetate [FDA (10μg/ml) (Sigma Aldrich, India)] for 15 min and non viable cells were counter stained with propidium iodide [PI (10μg/ml) (HiMedia, India)] for 1 min. The cells were visualized under a fluorescence microscope (DMI 6000E, Leica, Germany) using filters I3/N21.
Cells cultured on test materials and control glass cover slip were evaluated by rhodamine phalloidin (Invitrogen, USA) staining. After 48 h the cell monolayer was washed thrice with phosphate buffered saline (PBS) and fixed in 4 % par formaldehyde for 2 h. The fixed cells were rinsed with PBS, and permeabilised with 0.1 % triton X-100 in PBS for 3 min. Subsequently triton X-100 was removed by rinsing thrice with PBS. Rhodamine conjugated with phalloidin (1:1000) was added to the cells and incubated for 15 min. This was followed by counter staining nucleus with Hoechst 33258 for 1 min. Cells were visualized under a fluorescence microscope (DMI 6000E, Leica) using filters N21/A.
For SEM analysis of cell adhesion, samples were fixed by 2.5% glutaraldehyde in phosphate buffer followed by rinsing with PBS. After rinsing, the samples were dehydrated in graded concentration of ethanol and gold sputter coated before proceeding for SEM (FEIQuanta 400, Netherlands) imaging.
The cell activity was calculated by quantifying the metabolic activity through MTT [3-(4,5- dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide] assay. The test materials after 48 h of cell adhesion were analyzed by MTT assay. Samples were rinsed thrice with medium and 400 μl of MTT solution (1 mg/ ml MEM without serum) was added and incubated for 2 h. After 2 h the formazan crystals formed were solubilised by 800μl of isopropanol and transferred to a 96 well plate. The optical density (OD) of the solution at 570 nm was measured using a UV/Visible spectrophotometer (Power Wave XS, Biotek, US). Cell activity was calculated as the percentage relative to the untreated control cells using the equation, Cell activity (%) = (OD test cells/OD control) × 100. (1)
Statistical significance was determined using Student’s t test. P-values less than 0.05 were considered as statistically significant.
The various concentrations (wt. %) of 45PCL and 10PCL that were attempted to be electrospun are shown in Table 1. It was observed that the regularly reported concentration of 8-12 wt. % for Electrospinning 80PCL was not sufficient for Electrospinning of both 45PCL and 10PCL. Although 45PCL formed bead free fibres around 20 wt. %, 10PCL was not electrospinnable up to even 30 wt%, beyond which the solution was not process able. Hence 10PCL was not used for the Electrospinning of composite mats. The 20 wt. % of 45PCL was used for the forming the composite mats. A 45PCL/ HA of 90/10 was found to be the ideal ratio for Electrospinning of the composite mat. The optimized and electrospun 45PCL and 45PCL/HA mats will be referred to as LPCL and LPCLHA while the electrospun 80PCL and 80PCL/HA mats will be referred to as PCL and PCLHA respectively (Table 1).
Table 1: Various Polymer Concentrations, Flow Rate, Voltage of 45PCL and 10PCL.
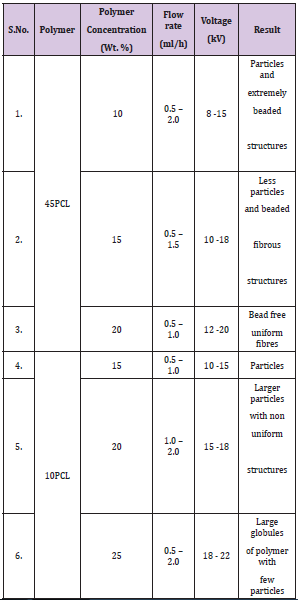
The surface morphologies of the electrospun mats as observed under a SEM are shown in (Figure 1). It was observed that LPCL and LPCLHA had fibres in the range of 310±50 nm and 400±100 nm, while PCL and PCLHA had larger diameter fibres in the range of 550±120 and 640±165 nm respectively. In both molecular weight regimes, the effect of HA seems to be the same in increasing the overall diameter of the fibres. This is generally observed in Electrospinning of composites [16]. The corresponding EDS patterns are shown as inset in the images. Incorporation of HA in the composite mats is evidenced by the presence of Ca and P elements (Figure 1).
Figure 1: SEM images of surface of a) LPCL b) LPCLHA c) PCL and d) PCLHA. The corresponding EDS patterns are shown in the respective insets.
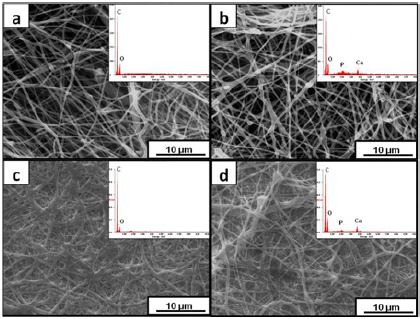
Figure 2: XRD patterns of the electrospun mats and HA.
The XRD patterns of the electrospun mats and HA are shown in (Figure 2). Crystalline PCL has an orthorhombic crystal structure with XRD peaks around 21.7° and 23.7° along with a smaller shoulder at 22° corresponding to the (110), (200) and (111) planes. These were observed for all the electrospun mats. The pattern obtained for HA was indexed according to JCPDS file no. 9-432. The high intensity peaks of HA were visible in the patterns obtained for the composite mats, thus confirming their presence in the fibers (Figure 2).
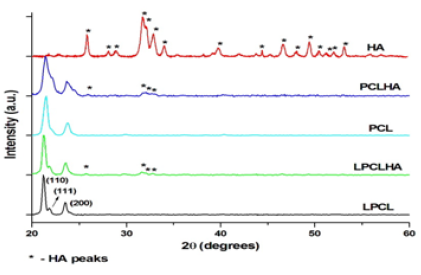
The FT-IR spectra of the electrospun mats and that of HA are shown in (Figure 3). All the electrospun mats display infrared bands related to the stretching modes, namely asymmetric CH2 stretching (2923 cm-1), symmetric CH2 stretching (2857 cm-1), carbonyl stretching (1720 cm-1), C-O and C-C stretching in the crystalline phase (1293 cm-1) and asymmetric COC stretching (1240 cm-1) corresponding to PCL. CH2 bending vibrations at 1464, 1418, 1397 and 1366 cm-1 were also observed. The spectra of HA displays all functional groups characteristic of HA [15]. In both the composite mats, the vibration bands corresponding to (PO4)3-bending at 1023 and 560 cm-1 of HA were also observed. Thus, the FT-IR spectra confirm the functional groups of the polymer and ceramic as well as the incorporation of HA in the composites (Figure 3).
Figure 3: FT-IR spectra of the electrospun mats and HA.
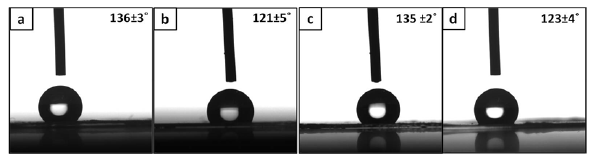
Figure 4 shows the results of contact angle analysis performed on the electrospun mats. All the contact angles are obtuse due to the hydrophobic nature of PCL. It is interesting to see that the contact angles on the composite mats are lesser when compared to their polymer counterparts (Figure 4).
Figure 4: Images of a water droplet on a) LPCL b) LPCLHA c) PCL and d) PCLHA. The contact angle made by the droplet is also mentioned in the image.
The weight loss with time is shown in Figure 5a. It is seen that LPCL and LPCLHA degrade at a faster rate than PCL and PCLHA respectively. Thus, the presence of HA has accelerated the degradation process. Though initially PCLHA degrades slower than LPCL, after 2nd week it degrades faster than LPCL. PCL electrospun mat maintained its structure, although with considerable weight loss, even after 6 weeks. LPCLHA displayed complete disintegration after 4th week, while PCLHA and LPCL lost their integrity after 5th week. The variation in pH of the PBS containing the electrospun sample mats is shown in Figure 5b. All samples show continuous decrease in pH with the increase in time. While LPCL shows the lowest pH by the end of 6 weeks, PCLHA shows the highest pH. Initially pH decrease in PCL is not as prominent as in LPCL. However after 2nd week, pH decreases more rapidly for PCL. This can be linked to the slower degradation of PCL compared to LPCL. Even though there is rapid degradation in PCLHA after 2nd week, the corresponding change in pH is not abrupt. By 4th week, the composites have a less acidic pH than the polymer samples which maybe a consequence of HA particles released due to degradation of the mats (Figure 5).
Figure 5: Weight loss (a) and change in pH, (b) with time during degradation.
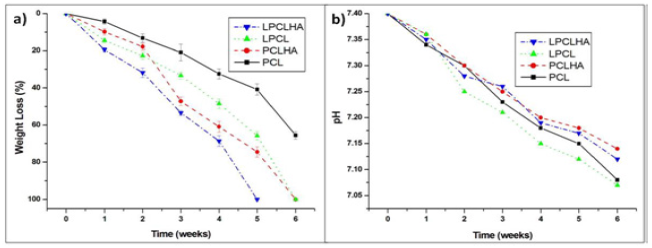
Figure 6: Live/Dead image of (a) control coverslips (b) LPCL (c) LPCLHA (d) PCL and (e) PCLHA. First image represents fluorescence from live cells second shows signal from dead cells and third is the merge of first and second images.
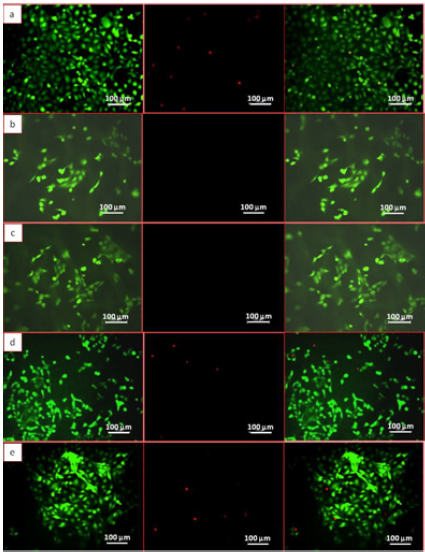
Figure 7: Actin phalloidin image of (a) control cover slips (b) LPCL (c) LPCLHA (d) PCL and (e) PCLHA. First image represents fluorescence from actin, second shows signal from nucleus and third is the merged images.
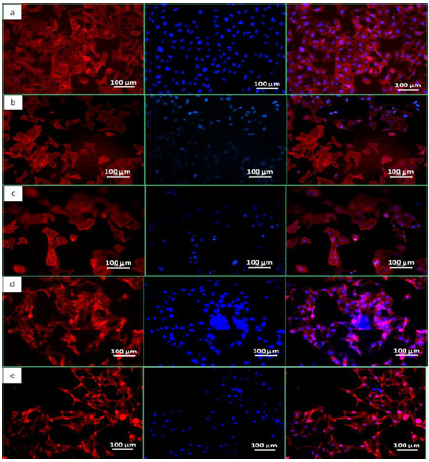
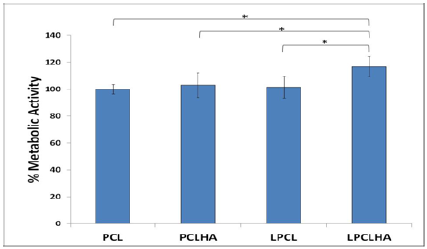
Fluorescence images of the HOS cell populations on the control and test samples, with the cytoplasm of the live cells stained with FDA (green), the nucleus of the dead cells stained with PI (red) and the respective overlay, are shown in (Figure 6). The Live/ Dead assay distinguishes live cells by the presence of intracellular esterase activity by hydrolysis of FDA. After 48 h of incubation, a high density of live cells was revealed on all the samples, depicting the non cytotoxic nature of the materials (Figure 6). The fluorescent microscope images of the stained HOS cells adhered on the electrospun mats are shown in (Figure 7). The rhodamine stained actin filaments and the Hoechst 33258 stained nucleus were visible in the control and test samples. While the cells seem to be well grown and adhered on all the samples, the cells on LPCL and LPCLHA seem to be a more spread out than on PCL and PCLHA mats (Figure 7). Figure 8 shows the SEM analysis of the cells adhered on the electrospun mat. HOS cells were adhered and spread well on all the materials implying that the electrospun mats support cell growth (Figure 8). Cytocompatibility was evaluated by MTT assay. The cell metabolic activity normalized with cell control is shown in (Figure 7). It is seen that all the electrospun mats display a metabolic activity of 100% or more. LPCLHA shows the maximum metabolic activity and is also significantly higher than the rest of the samples (Figure 9).
Figure 8: SEM images of the cells proliferated on a) LPCLn b) LPCLHA c) PCL and d) PCLHA mats.
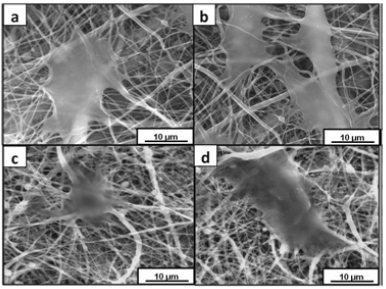
Figure 9: Cell metabolic activity measure on the electrospun samples.
The 45PCL concentration necessary to form an electrospinnable solution was higher (20 wt. %) than what is reported for 80PCL (8- 12 wt. %). In general Electrospinning of a polymer solution occurs when there are enough chain entanglements [14]. Also for a given MW entanglement density increases with increasing concentration. Hence it can be said that the critical entanglement density necessary for Electrospinning the 45PCL was achieved at 20 wt%. For preparing the composite mat, it was seen that a HA wt. % of more than 10 resulted in beaded fibres. Moreover LPCL solution results in fibres of diameters lesser than PCL and PCLHA respectively. It may be said that even when using a higher concentration, a solution made of lower MW polymer results in finer fibres than when using a higher MW of the same polymer. A detailed study of parameters such as viscosity, electrical conductivity and their interdependence is needed for better understanding of the reason for such behavior.
Both XRD and FT-IR analyses confirm the incorporation of HA in the composite electrospun mats. Based on literature and the results obtained from degradation studies, a schematic of the progress of degradation is shown in (Figure 10). As expected, lowering the MW has increased the degradation rate. Additionally the presence of HA has also accelerated the degradation. This can be attributed to the hydrophilic nature of HA, which facilitates easier and quicker penetration of water into the composite, consequently accelerating the hydrolysis reaction that occurs during PCL degradation [2]. Similar effect of HA in a PCL matrix during degradation has been reported [17].
Figure 10: Schematic of the degradation occurring in PBS medium with lipase enzyme for a)PCL b) PCLHA c) LPCL and d) LPCLHA.
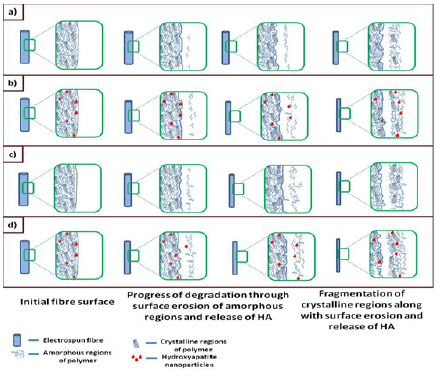
Since degradation of PCL proceeds through surface erosion, there is also the possibility of the gradual release of HA nanoparticles that are embedded in the composite mats. Thus release of HA is also contributing to weight loss. Also since PCL degrades slower than LPCL, the release of HA from PCLHA may have also occurred later than that of LPCLHA. This is seen (Figure 5a), where weight loss in PCLHA overtakes LPCL, due to the onset of release of HA after 2nd week. The faster degrading low Mw PCL in LPCL and the presence of HA in PCLHA had accelerated the degradation and resulted in their structural disintegration by 5th week. Both the mentioned factors were present in LPCLHA which resulted in it degrading and losing its structural integrity earlier by 4th week.
Thus the faster degrading nature along with the presence of HA could have resulted in LPCLHA showing the highest cell metabolic activity and widespread cell adhesion and proliferation (Figure 10). Rapid disintegration of polymers has the tendency to induce inflammatory reactions due to the acidic by-products [18]. Although slow degrading PCL has not been reported to cause any such major issues, this issue may arise with the relatively faster degrading LPCL. However the addition of HA to LPCL seems beneficial due to their buffering action [7]. As seen in (Figure 5), even though the LPCLHA has the highest degradation rate, the corresponding decrease in pH is the lesser than the polymer mats. This also seems to be true with PCLHA.
It was hypothesized that, if chain entanglements necessary for Electrospinning were achieved, even low MW PCL can be electrospun. Though this was possible with the 45PCL, with 10PCL Electrospinning was not possible even at high solute concentrations. The 45PCLHA was found to degrade faster in PBS buffer than the 45PCL, 80PCL and 80PCLHA mats. The electrospun mats were found to be cytocompatible and suitable for growth of osteoblast like cells. Hence investigations on the Electrospinning of low MW varieties of PCL can result in widening the scope of applications of PCL and PCL based composite scaffolds in bone tissue engineering.


