Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Msetule L1, Komba EVG2*, Kimera SI3 and Mdegela RH3
Received: September 12, 2017; Published: October 03, 2017
Corresponding author: Komba EVG, Department of Veterinary Medicine and Public Health, College of Veterinary Medicine and Biomedical Sciences, Sokoine University of Agriculture, Tanzania
DOI: 10.26717/BJSTR.2017.01.000406
Purpose: Majority of the human bacterial gastroenteritis cases in both developed and developing countries in the world are caused by thermo tolerant Campylobacter spp, with C. jejuni and C. coli being more involved. These bacteria live in poultry and other animal’s intestinal tracts without causing disease symptoms. Improper handling of carcasses during slaughter and evisceration increases the chance of contaminating the outer skin. Poor meat handling in the kitchen and poor storage during refrigeration causes cross contamination.
Methods: This cross-sectional study examined the occurrence of antimicrobial resistant thermophilic Campylobacter in frozen chicken meats in Morogoro Municipality. A total of 272 frozen broiler chicken carcasses were obtained from supermarkets (n=90), meat shops (n=90) and restaurants (n=92). Each carcass was divided into three parts i.e. breast, thighs and wing+neck, rinsed in peptone water. The rinses were then enriched in 5ml of Bolton broth in microerophilic atmosphere at 42ºC for 24hrs. Then the broth was streaked on Modified Charcoal Cefoperazone Deoxycholate Agar (mCCDA) plates followed by incubation at 42ºC for 48 hrs. Presumptive colonies of Campylobacter spp. were sub cultured onto Mueller Hinton Agar and incubated at 42ºC for 48 hrs to obtain pure colonies. Pure Campylobacter colonies were then subjected to preliminary identification using biochemical tests and further confirmed by polymerase chain reaction (PCR). Antimicrobial Susceptibility testing was performed on C. Jejuni isolates by disc diffusion method on Mueller-Hinton agar supplemented with 5% of horse blood. The isolates were tested for resistance against ten antimicrobials namely tetracycline, gentamycin, ciprofloxacin, azithromycin, erythromycin, norfloxacin, chloramphenical, amoxicilin, nalidixic acid and cephalocin.
Results: Occurrence of thermophilic Campylobacter spp. in the sampled birds was at a tune of 61.0%. Based on sampling locations the levels of contaminated chicken carcasses were 82.6%, 62.0% and 37.8% for restaurants, shops and supermarkets, respectively. Majority of isolates (72.4%) were Campylobacter jejuni and the remaining proportion (27.6%) was accounted for by C. coli. Frequencies of contamination were comparable between wing+neck and thighs. Wing+neck and thighs had significantly higher frequencies of contamination than breasts (P<0.05). Antibiotic resistance test results for C. jejuni indicated that higher levels (>80%) of resistance were observed for cephalothin, chloramphenical, nalidixic acid, amoxicillin and tetracycline. Lower levels of resistance (<15%) were observed for erythromycin, norfloxacin, azithromycin, ciprofloxacin and gentamicin.
Conclusion: This study confirms that in our setting a high frequency of commercial broiler chickens are positive for antimicrobial resistant Campylobacter at the time of slaughter. This phenomenon derives in high contamination of carcasses during the slaughter process thereby constituting a substantial public health hazard. Freezing of carcasses does not completely remove this bacterium as seen from this study. Control strategies for these pathogens at flock level are recommended to avoid contamination of the final product. Animal products should be properly handled and thoroughly cooked in order to make sure that safe products are consumed.
Keywords: Broiler meat; C Coli; C Jejuni; Restaurants; Supermarkets; Tanzania
Abbreviations: Mccda: Modified Charcoal Cefoperazone Deoxycholate Agar; PCR: Polymerase Chain Reaction
Thermo tolerant Campylobacter spp. in particular C. jejuni and C. Coli, are among the leading causes of human bacterial gastroenteritis in the world [1-4]. Of the two species, C. Jejuni is responsible for majority (80-90%) of the food-borne Campylobacter infections [5]. The organisms primarily colonize the small intestines of a wide variety of animals, avian being the most common reservoirs [6,7]. The frequently mentioned risk factor for human infections is consumption of raw or undercooked poultry meat [8,9]. Other food products can serve as sources of human infection through crosscontamination [10].
A large number of Campylobacter spp. is harbored by the intestinal tract of chicken, especially the ceca and colon. During processing activities, especially during evisceration where the tract may leak or rupture, its contents can easily contaminate chicken carcasses, surface water and environments [11,12]. During slaughter, contamination of chicken carcasses can also occur indirectly through contaminated equipment and water. As a survival mechanism, campylobacter’s become trapped in folds and crevices of the skin particularly in the feather follicles [13]. As a result several studies, most of them conducted in developed countries, have shown that there is a high prevalence of Campylobacter in raw poultry meat and more than one species can be present in a sample [14,15].
Studies elsewhere have demonstrated that Campylobacter survive in raw and cooked poultry meat during refrigerated or frozen storage [12,16-18]. The studies report frequent isolation of C. jejuni and C. coli in retail chicken carcasses [14], with the former occurring more. The aim of this study was therefore to establish the contamination levels of frozen broiler meat with thermophilic Campylobacter organisms and determine antimicrobial resistance profiles of the associated isolates in Tanzania. Results of the study complement findings in most of case control studies that report consumption of poultry meat to be among the potential risk factors for human Campylobacter infections.
The study was conducted in Morogoro Municipality, western Tanzania. Geographically the Municipality is located between longitude 35o15’ to 38o30’ East and latitude 5o15’ to 10o0’ South on slopes of Uluguru Mountains at an altitude of 526.0 m above sea level. It has a bimodal rainfall pattern with 188.5-409.4 mm and temperature ranges from 15.0 to 33.1oC. The Municipal is featured by backyard exotic chicken production involving both broilers and layers. At slaughter age broilers are either bought on retail basis by consumers at farm gate or whole sale basis by supermarket, butchers and restaurant owners; with the birds being slaughtered at the farm.
A cross sectional study design was adopted. The study involved collection of frozen chicken samples from meat shops, supermarkets and restaurants. The sample size was estimated by using the formula N = Z2P (1-P)/d2 Martin et al. [19]. Where by N= is the sample size, Z= is a multiplier from the normal distribution i.e. 1.96, P= is expected/known prevalence and d2= is degree of accuracy desired (0.05) with a 95% confidence interval. Based on a previous study done in a nearby country of Kenya [20], a known prevalence of 77% was used. Using the above formula a sample size of 272 chicken carcasses was obtained. Simple random sampling was adopted. During sampling dressed chicken carcasses were handled in individual clean plastic bags. Following collection the samples were immediately conveyed to the laboratory on ice.
Sample Processing and Enrichment Procedures
The detection of thermo tolerant Campylobacter in foods requires selective enrichment broths at 42ºC under microaerophilic atmosphere [21] thus providing adequate conditions for growth of thermo tolerant species, protection against toxic oxygen derivatives and recovery of injured cells [22]. In the laboratory each frozen chicken carcass was cut into three portions i.e. wings+neck, thighs and breasts. The portions were then rinsed with 200mL bufferedpeptone water (Oxoid Ltd, Basingstoke, U.K.) and then gently shaken for 1 min. For the test, 25mL from the 200mL rinsate and 25mL of 2 × Bolton broths (Oxoid Ltd, Basingstoke, U.K.) were mixed and then incubated at 42°C for 24h under Micro-aerobic condition.
Isolation of Campylobacter Spp.
Following shaking, a loopful of enrichment was streaked on mCCDA agar plates (Oxoid Ltd, Basingstoke, U.K.) for primary isolation of thermophilic Campylobacter. The agar plates were incubated at 42ºC under microaerobic condition for 48hrs. Presumptive Campylobacter colonies were subculture onto Mueller- Hinton agar supplemented with preston Campylobacter selective supplement (Oxoid Ltd, Basingstoke, U.K.) and then incubated at 42ºC for 48hrs under microearobic condition.
Preliminary Identification
Presumptive identification of thermophlic Campylobacter spp. was done by traditional morphological methods like colonial characteristics and microscopy (Motility and Gram Staining); and Biochemical Methods (oxidase and catalase reactions, hippurate hydrolysis, and susceptibility to Cephalothin and Nalidixic acid [23,24].
Polymerase chain reaction was used for definitive identification of the Campylobacter spp. C. jejuni and C. coli were identified using species specific PCR adopting a method described earlier by Nachamkin et al. [25]. Genomic DNA to be used for PCR was extracted from bacterial suspensions by boiling at 100°C for 10min. Primers F,5’CTATTTTATTTTTGAGTGCTTGTG3’ and R,5’GCTTTATTTGCCATTTGTTTTATTA3’ (TAG COPENHAGEN A/S, Denmark) were used to amplify the mapA gene of C. jejuni, where as primers F,5’ATTTGAAAATTGCTCCAACTATG3’ and R,5’TGATTTTATTATTTGTAGCAGCG3’ (Tag Copenhagen A/S, Denmark) were used to amplify the ceuE gene of C. Coli. Each reaction was performed in a 50μl total volume containing 10μl primer mix (12pmol of each primer), 25μl Green master mix, 2μl DNA template and 13μl of nuclear free water. Amplification reactions were done by using an automated thermal cycler machine (Crocodile II Appligene Inc. Pleasanton CA, USA). The amplification process started with denaturation at 94oC for five minutes, then 35 cycles at 92oC for 30 seconds followed by annealing at 55oC for one and half minutes and extension at 72oC for two and half minutes, then incubated at 72oC for 5 minutes and maintained at 4oC until analysed. The amplification generated 589bp and 462bp DNA fragments corresponding to Campylobacter jejuni and Campylobacter coli respectively. The PCR products were analyzed on a 0.8% agarose gel stained with 0.3g/ml ethidium bromide and were visualized under UV light. A 100bp ladder was used as a molecular size standard.
Campylobacter isolates were tested for resistance to different antimicrobials by the disc diffusion method on Muller Hinton Agar (Oxoid Ltd, Basingstoke, UK) supplemented with 5% of horse blood. Briefly, bacterial suspensions were prepared in a sterile normal saline and adjusted to a turbidity equivalent to 0.5 McFarland standards. The suspensions were inoculated onto Mueller-Hinton agar plates and dried; and then antibiotic discs distributed over the inoculated plates using a BBL Sensi-disc dispenser (Oxoid Ltd, Basingstoke, UK). The plates were then incubated at 42°C for 48 hours under microaerobic conditions. After 48 hours diameters of inhibition zones were measured and results interpreted in accordance with interpretive criteria provided by NCCLS [26] and manufacturer’s instructions. Sixty Campylobacter isolates were tested for resistance against the following antimicrobials; ciprofloxacin (CIP, 5μg), Gentamycin (CN, 10μg), Amoxycillin (AML, 25 μg), Norfloxacin (NOR, 10μg), Erythromycin (E,15μg), Tetracycline (TE, 30μg), Azithromycin (AZM, 15μg) and Chloramphenicol (C, 30μg) (Oxoid, Hampshire, UK).
Collected data in this study were first entered and cleaned in Microsoft Office Excel® 2007 (Microsoft Corporation, One Microsoft Way, Redmond, 98052-7329, USA) and imported into Epi- Info version 7 (CDC Atlanta, USA) for analysis. Contamination levels of different parts of the carcasses were determined by computing descriptive statistics. Differences in the contamination levels were determined by computing the chi square. A p value of 0.05 or less was considered statistically significant.
A total of 272 frozen chicken carcasses were collected from different sources in the following order, shops (n=90), supermarkets (n=90) and restaurants (n=92). Storage of the carcasses differed from one source to another. In shops and restaurants all carcasses were pooled together in freezers at -20ºC; whereas in supermarkets each carcass was packaged in a clean plastic bag and stored in freezer at -20ºC. Overall 61.0% (n=166/272) of frozen chicken meat samples were found to be contaminated with Campylobacter spp. as noted by growth of characteristic colonies on mCCDA and on MH agar. According to the sources of the samples the frequencies of chicken carcass contamination were 62% for shops, 37.8% for supermarkets and 82.6% for restaurants. The differences in these frequencies of Campylobacter detection were statistically significant at a p-value≤0.05.
Figure 1: Frequencies of contamination of Different Portions of Broiler Thermophilic Carcasses with Campylobacter.
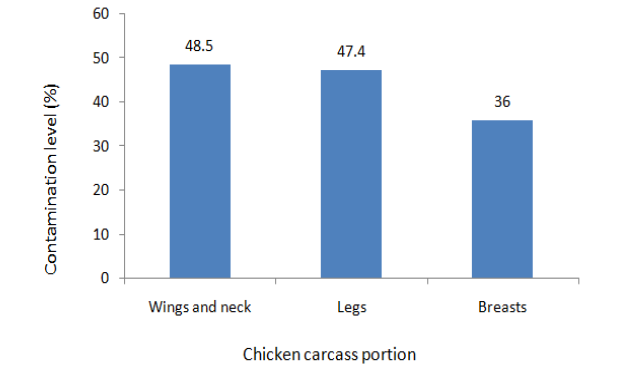
Contamination, of different chicken carcass portions, with thermophillic Campylobacter spp. ranged from 36.0% to 48.5% (Figure 1). Contamination levels were similar for wings and thigh. The contamination levels of these portions were however significantly higher (p≤0.05) when compared to that of breasts.
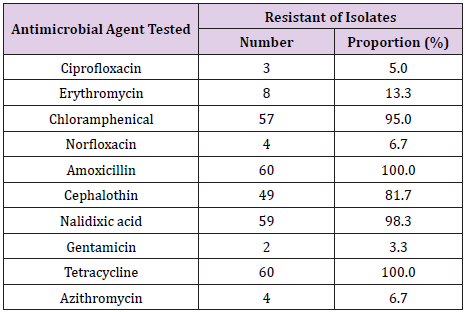
A total of 60 Campylobacter Jejuni isolates were tested for resistance against ten antimicrobial agents. The levels of resistance among the isolates ranged from 3.3% to 100.0% (Table 1). The highest resistance levels were observed for amoxicillin and tetracycline, whereas the lowest resistance level was recorded for Gentamycin.
Table 1: Antimicrobial resistance profiles of 60 Campylobacter Jejuni isolated from frozen chicken carcasses in Morogoro,Tanzania.
The current study reveals occurrence of thermophilic Campylobacter spp. in frozen broiler meat. There are several other reports describing Campylobacter spp. contamination in frozen retail poultry meats and/or by-products in the world in spite of their sanitary conditions [15]. Such reports, from both developed and developing countries, reveal that poultry meat is the food vehicle most frequently contaminated with Campylobacter spp. [27]. These findings, along with the findings in this study are suggestive of high prevalence of Campylobacter at flocks level as previously reported by Stern et al. [28] and Arsenault et al. [29] who observed a positive correlation between the contamination of carcasses and the high positivity rates for Campylobacter of flocks at the farm level. The ability of Campylobacter spp. to survive refrigeration and freezing has a huge implication to food safety and public health given that ingestion of only 500C. Jejuni cells has resulted into illness in human experimental infections [30,31].
The prevalence of Campylobacter contamination on poultry products worldwide is reported to be in the range of 0 to 100% [15,32,33]. The obtained prevalence of frozen chicken contamination in this study (61.0%) falls within this stated range. More or less similar contamination prevalence’s have been reported in Brazil 62.2% [34] and in Iran 63.0%; [27]. Low prevalence’s of retail poultry contamination with Campylobacter spp. have been reported in Brazil 47.5%; [35], Japan 45.8%; [36], Chile 45% [37], USA 41.0% [38] and in Bulgaria 35.2% [39]. Some years back Lee et al. [40] showed that C. Jejuni was able to survive for up to almost two months under frozen storage at -20ºC, which is the temperature found in most domestic freezers. A more or less similar observation was made by Georgsson who demonstrated survival of Campylobacter spp. in chicken meat stored at -20ºC for 31 days. As several studies demonstrated that Campylobacter survive in raw and cooked poultry meat during refrigerated or frozen storage [12,16,17,18,40], frozen storage of foods cannot be considered a safety assuring procedure. Studies however report that frozen poultry meats and by-products have shown lower prevalence compared with poultry meats and by-products. This can be attributed to the fact that frozen conditions damage Campylobacter spp. cells and decrease their viability [17,41].
In the present study samples were collected from meat shops, restaurants and supermarkets. In meat shops and restaurants carcasses were found to be stored in freezers pooled together providing a room for cross contamination. This was reflected in the prevalence of positive chicken carcass samples detected from the different sources, being significantly higher for those from shops and restaurants. This further explains possibilities for cross contamination of food products either during storage or preparation in the kitchen once hygienic practices are not observed.
We noted in this study that C. Jejuni was the predominant thermophilic Campylobacter species responsible for causing contamination of poultry meat. This observation is similar to what has been observed in other studies elsewhere in which authors reported C. Jejuni to be the most prevalent thermophilic Campylobacter spp. in chickens than others [42-46]. Some other studies have however reported findings contrary to the current observation. These include a study in Thailand in which the authors found that C. coli were more frequently isolated from retail poultry meats and by-products compared to C. jejuni. Two different studies in the same country found that C. coli and C. jejuni were almost comparably prevalent in poultry at farms, with the ratio of C. coli to C. jejuni however drastically increasing via processing plants to markets [47,48]. The authors suggested the possibility of transmission of Campylobacter spp. from non-poultry sources to poultry, especially at markets. Fernández and Pisón [49] who also found a higher prevalence of C. coli as compared to C. jejuni in chicken liver samples suggested that C. coli could be more resistant to injury resulting from exposure to low temperatures and adverse ambient conditions.
During collection of swab samples chicken carcasses were portioned into wings and necks, breasts and thighs. Culture results indicated that wings and necks; and thighs had higher levels of contamination with Campylobacter spp. than breast samples. This observation could be linked to the fact that during the chicken slaughter process, particularly during the process of evisceration, most people prefer to hold wings, neck and thighs. For that matter, once unfortunately there is rupture of intestines followed by fecal contamination to people hands these areas preferred for handling easily get contaminated. A similar finding to what was observed in this study was reported in Bulgaria in which 35.2% of frozen poultry carcasses were contaminated by Campylobacter spp. with high percentages of 91.1% and 88.9% detected in the wing and thigh cuts, respectively [39]. Further, our results are supported by a study done in California USA in which the authors found a higher contamination with Campylobacter jejuni of chicken wings purchased from retail outlets on the day of arrival at the supermarket [50]. Other authors cautioned that high Campylobacter load in chicken wings could increase the probability of pathogen transfer to other surfaces through cross contamination and inappropriate handling during meal preparation and cooking [51]. It has been suggested earlier that higher Campylobacter contamination levels in chicken wings might be attributed to imperfect scalding, postscalding contamination, or due to the combination of both [52].
Worldwide reports indicate that antimicrobial resistance is increasing in food, food animals and human bacterial isolates; including Campylobacter [53-57]. Campylobacter isolates recovered from frozen broiler meat in the present study demonstrated varying proportions of resistance to different antimicrobials; ranging from 3.3% to 100.0%. Higher levels of resistance were noted for Amoxicillin (100.0%), Tetracycline (100.0%), Nalidixic acid (98.3%), Chloramphenical (95.0%) and Cephalocin (81.7%) whereas the lowest resistance level (3.3%) was recorded for gentamycin. Similar, lower and higher frequencies of resistance to these different antimicrobial agents have been reported in other studies [14,46,58-64].
Some C. Jejuni isolates in this study were resistant to ciprofloxacin and erythromycin, the known to be drugs of choice for treatment of cases of human campylobacteriosis that require therapeutic intervention i.e. persistent or complicated cases and those involving immuno-compromised individuals [65]. Resistance to these two antimicrobial agents was low in this study indicating that they may still be useful in the treatment of human campylobacteriosis in the country. A similar observation of low resistance to ciprofloxacin among thermophilic Campylobacter isolates was reported in a recent studies conducted in the country [9,66]. The authors however found higher frequencies of resistant isolates to erythromycin which is contrary to what we report in this study. Studies in Nigeria [67,68] reported that ciprofloxacin was effective against all the strains of Campylobacter tested. Many other studies have revealed variability in proportions of resistant Campylobacter isolates to ciprofloxacin and erythromycin [57,69- 73]. Beatty et al. [74] reported that the resistance of Campylobacter to erythromycin is increasing and varies between 12% and 95%.
All the C. jejuni isolates obtained in this study were resistant to amoxicillin and tetracycline. A higher level of resistance to tetracycline among C. Jejuni isolates was reported previously in the country [75]. Recent studies in the country have however reported a moderate frequency of tetracycline resistance among thermophilic Campylobacter isolates [9,66]. In these studies however the authors reported higher resistance levels to nalidixic acid among Campylobacter isolates which is in line with this study. Tetracyclines are the most extensively used drugs in veterinary medicine in Tanzania. In Canada, Gaudreau and Gilbert [57] reported that the rate of resistance of C. Jejuni to tetracycline rose from 19.1% to 55.7% in a period of 10 years. Tetracyclines are known to be relatively cheap and have a broad spectrum of activity. For this reason, they have been broadly used in the prophylaxis and therapy of human and animal infections and to promote animal growth.
The current study reveals contamination of frozen broiler meat with antimicrobial resistant thermopilic Campylobacter.
We recommend control plans for the pathogens at all levels from farm to table so as to stem human infections attributable to broilers as sources. Further research is needed to investigate the physiological mechanisms underlying the ability of C. Jejuni to survive following prolonged exposure to low temperatures in spite of being very fragile and fastidious. Variations in resistance observed over time underscore the need for continued public health monitoring of Campylobacter resistance from humans, animals, and food [76].


