Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
SA Lone1*, AR Paray2, SH Mir3, BA Ganaie1, R Sinha2 and P Singh2
Received: September 22, 2017; Published: October 05, 2017
Corresponding author: SA Lone, Animal Reproduction, Gynecology & Obstetrics, ICAR- National Dairy Research Institute, Karnal, 132001, Haryana, India
DOI: 10.26717/BJSTR.2017.01.000415
Breeding soundness evaluation (BSE) of bull is an easy, cheap, and an essential tool for the cow-calf operation. BSE reduces risk, improves, strategic bull usage, fertility of herd and economics. A field practitioner can play an important role in achievement of whole potential of BSE by performing it properly. A properly performed BSE should include the use of Society for Theriogenology based standards and a systematic protocol by the practitioner. Proper Semen evaluation is an important component of the BSE. Competent physical/reproductive exams and appropriate semen evaluations can contribute greatly to the fertility and economics of individual herds as well as understanding of factors which affect fertility.
Keywords: Bull; Breeding Soundness; Semen Quality
Bull breeding soundness evaluation (BSE) in is a procedure which reduces risk and improves strategic bull usage and herd fertility. The breeding soundness evaluation (BSE) is a method to evaluate the potential of a bull to be used as herds sire [1]. One of the essential components of the BSE is accurate semen evaluation. Standardized procedures and assessments for bull breeding soundness evaluation (BBSE) were first brought by the precursor of the American Society for Theriogenology (SFT) [2]. Today, various bull evaluation systems include a systematic physical examination which focuses upon the assessment of sperm motility, morphology and other reproductive functions [3]. Different surveys in a variety of locations and environments revealed that subjecting bulls to a BSE may help in classification of around 65–85% of bulls as “satisfactory potential breeders” [3,4]. However, this figure can vary with bull ages, genotypes, genetics, environment, management, prior selection and the particular BBSE criteria employed [5].
Study revealed that bulls which pass a BBSE and/or related semen quality tests, have a 6% or higher fertility as compared to unevaluated bulls [6]. Calf crops were higher when using bulls which had >70% normal spermatozoa and lowest from bulls with < 50% normal spermatozoa [7] concluding that semen quality, particularly normal spermatozoa percentage was consistently related to calf output. BSEs provided a benefit/cost ratio of approximately 36:1 in Brazil [8] and 17:1 in USA [9]. Using natural breeding bulls in dairy operations, the benefit/cost ratio of eliminating infertile dairy bulls was estimated at approximately 14:1 [10].
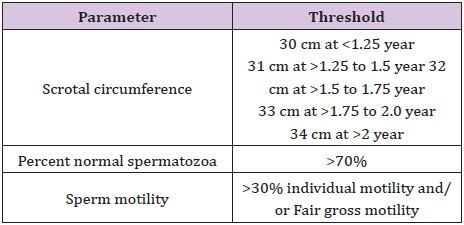
As per the Society for Theriogenology (SFT) a thorough knowledge of the minimum BSE standards is mandatory for performing a proper BSE. In order to categorize bulls as satisfactory potential breeders, they must pass the physical examination and equal or exceed the minimal thresholds in each of the categories mentioned in Table 1. Bulls which do not meet the thresholds mentioned in the Table will be classified as unsatisfactory potential breeders.
Table 1: Minimal thresholds of scrotal circumference, sperm motility and normal sperm.
Bulls approach should be watched in chute for gait and overall appearance of the bull. Once in chute, the identification, head, eyes and front feet are observed, and observations recorded. Testes palpation, scrotal circumference measurement, prepucial abnormalities (if any) and hind feet are observed from the side of the chute. Scrotal circumference and any observations are also recorded. After this rear of the bull is approached and back, hind limbs, especially the hock joints, are observed and/or palpated. Again sequence is followed for transrectal palpation. Forward to the seminal vesicles, pelvic area and urethralis muscle are followed which are palpated and massaged, the ampullae are palpated and massaged after locating the fornix of the seminal vesicles and as are the inguinal rings, pelvic lymph nodes, iliac lymph nodes, the kidney, and any viscera within reach. Seminal vesicles and ampullae are massaged again. While progressing toward the anus, urethralis muscle and the prostate are identified and massaged. Besides, identifying abnormalities, bull is stimulated and ejaculation is facilitated by palpation and massage.
Thereafter, the electro-ejaculator is standardized and the probe is inserted into the rectum. Just after erection and protrusion, the internal prepuce and the penis are examined by observation and palpation. Some seminal fluid is collected in a pre-warmed StyrofoamTM cup before the collection of ejaculate. Immediately after collection, a drop of semen is placed on a clean grease free prewarmed slide and observed first for mass motility or gross motility and then for individual progressive motility. Results are recorded and then a slide is made for morphological evaluation using one drop of semen and one drop of eosin–nigrosin stain. If no further collection is needed, the bull is then allowed to move out from the chute. The bull leaving the chute is observed for lameness. Then another bull is brought into the chute and the next examination is initiated. Utilizing a second veterinarian, who remains at the microscope to perform semen evaluation and further minimizes the time in the chute for the bull, and makes the testing protocol more efficient. For bulls to be classified as Satisfactory Potential Breeders, they must pass the physical examination and equal or exceed the minimal thresholds in each of the following.
Bull semen evaluation served as a major component of the BSE since the 1950s, when the reliable electro-ejaculators played an important role in safe, routine, semen collection from unhandled bulls. Sperm live/dead estimation and concentration have now been deemphasized; those for sperm motility and morphology have increased in importance. Both sperm motility and morphology may be adequately assessed under field conditions provided appropriate preparation and precautions are applied [2,11]. More sophisticated technologies such as computer assisted sperm analysis (CASA) and differential-interference contrast (DIC) microscopy are now available for greater reliability [12]. These techniques can be used for sperm morphology assessments, Assessment and quality assurance (QA) for frozen/chilled semen, Investigating infertility problems, Collaborative and clinical research and Training of A.I. industry persons. Progress should be made in understanding pathogenesis, classification and significance of different types of spermatozoa defects encountered in bulls Brito [13]. Compensable sperm defects are those which interfere with spermatozoa from reaching the site of fertilization while as non-compensable sperm defects are associated with post-fertilization defects (such as embryo quality and loss).
Each bull is evaluated on all four categories of BSE standards and he must achieve at least the minimum in all four categories to be classified as a satisfactory potential breeder.
Examination is started from head of the bull, observe the eyes, for evidence squamous cell carcinomas, corneal damage, and lymphomas. Then front feet are observed. Screw claw and interdigital fibro as are common. Screw claw are prevalent in beef breeds and may be inherited, so such bulls should not be used as a purebred herd sire or his heifers should not be retained for breeding if he is used. Examination to the side of the bull, prepuce injuries, lymphomas, rear foot abnormalities, hernias, hock injuries, scrotal or testicular abnormalities or injuries can be detected and noted. Lymphomas, inguinal hernias, kidney abnormalities, adhesions, seminal vesiculitis, or prostate abscesses may be detected on transrectal examination. During penile protrusion and ejaculation, the penis and the internal prepuce are examined for tumors, lacerations, warts and scars which may interfere with coitus. Persistent frenulum is one of the more common defects detected in young bulls. Although this defect is usually corrected at the time of collection but it may have hereditary potential. Any single reason, or combination of reasons, may be responsible for classifying a bull as unsatisfactory potential breeder.
The scrotal circumference measurement is carried out during general and reproductive examination. Scrotal circumference measurement is an parameter for breeding soundness evaluation due to the fact that it assesses testicular volume and is highly correlated with sperm output. Considering that this measurement is accurate and easy to perform, it can be used for the selection of bulls at young age [14]. Depending individuals preference, the facilities and the bull’s temperament, scrotal circumference can be measured from the side or rear of the bull. Primary considerations after gently forcing both testes to the bottom of the scrotum are to avoid spreading the testes apart and that the top surface of the measure tape is even with the skin by ensuring sufficient pressure. Record the scrotal measurement; if the bull fails to meet minimum for scrotal circumference then there is no need to continue the examination [15].
In order to reduce cold shock, urine contamination, or other spermicidal contaminants, gross motility and individual progressive motility should be performed as soon after collection. In order to maintain maximum motility a microscope stage, glass slide and cover slip should be warm which can be achieved by heating pads, warmers, or slide warmers. Gross motility is observed microscopically with under low power (10×), whereas individual progressive motility is observed under 400×. Bulls with unsatisfactory un satisfactory motility are collected at least one additional time, to ensure that technique is not an issue. To be classified as a satisfactory potential breeder according to BSE minimum standards, the bull semen must have a minimum of 30% progressive motile spermatozoa or fair gross motility.
A satisfactory potential breeder bull should have >70% normal spermatozoa. Eosin–Nigrosin stained sperm smear is most common method of obtaining sperm morphology counts. Practically an oil immersion (1000–1200×), with either a bright-field or phasecontrast microscope is used. Sperm are classified as being normal or having primary abnormalities or secondary abnormalities. Morphology is done immediately after collection between bulls or after all bulls semen has been processed, depends on the number of bulls to be tested, and the availability of second veterinarian. Around 52% of unsatisfactory potential breeder bulls failed due to morphology provide ample evidence that without assessing sperm morphology; an adequate BSE cannot be carried out.
To the implement proven reproductive management procedures such as BSE, many challenges exist within the developing world [16]. Although the parameters employed may be debatable, the concept of an effective sperm dose (ESD) for frozen/thawed semen can be helpful for semen processors. Effective quality assurance (QA) is represented by accurate measurement of sperm numbers. Fertility may be compromised with low ESD, whereas a high ESD can prove uneconomical and inefficient in terms of usage of genetic material and fertility. Beggs [11] reported normal spermiogram was most likely associated with scrotal measurements between 31 and 44 cm. If scrotal circumferences in a given bull population follow a normal distribution then the bulls at the bottom of the curve are linked with decreased semen quality and/or fertility [17].
Natural breeding bulls are still employed on dairies despite the widespread use of A.I to achieve genetic progress in dairy cattle [3]. One of the neglected aspects of dairy management is proper selection and management of natural breeding bulls [18-20]. A number of positive implications are related to correlation between age at puberty in related females and bull scrotal circumference. In beef females, age at puberty is fertility and calf produced [18]. Selecting bulls for scrotal circumference and improved semen quality traits using BSE criteria can improve both immediate and future herd fertility and production.
BSE has been accepted as a standard for determining if a bull is a good potential breeder and a satisfactory method of identifying sub fertile bulls. Bulls will be examined and assessed efficiently with BSE minimum standards, a good examination procedure, and thorough understanding of spermatogenesis and sperm maturation process.


