Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Robert S Eisinger1* and Hans K Pilegaard2
Received: December 21, 2017; Published: January 08, 2018
Corresponding author: Robert S Eisinger, University of Florida College of Medicine, Gainesville, Florida 1275 Center Drive, Gainesville, FL 32611, Florida
DOI: 10.26717/BJSTR.2018.02.000640
Abbreviations: PE: Pectus Excavatum; HI: Haller index; CT: Computed Tomography
Due to increased awareness and recent advances in the minimally invasive repair of Pectus Excavatum (PE), a growing number of PE patients are considering corrective treatment. But patients and clinicians are faced with a pressing question: What are the indications for surgery? Due to the paucity of prospective PE studies, data-driven guidelines are currently lacking. The Haller index (HI) has emerged as the predominant way to identify patients for which surgical treatment is most appropriate. Under this premise, patients with higher HI values – indicating a more severe chest deformity – are more likely to pursue and/or benefit from surgery. However, evidence is accumulating that HI may not accurately portray the underlying physiologic disruption or the symptoms associated with PE. In this Viewpoint, we discuss the origin of HI and describe its widespread use in practice today, despite its possible shortcomings, as well as alternative approaches for evaluating PE severity.
Between 1983 and 1985, Alex Haller Jr. and colleagues from the Johns Hopkins Hospital examined computed tomography (CT) scans from 33 PE patients previously determined to require surgical correction [1]. These determinations were made on the “subjective basis of abnormal chest wall dynamics, general distortion of chest wall configuration, and caliper measurement of anterior-posterior chest diameters.” After computing a CT-scan derived HI for each patient, defined as the ratio of the transverse chest and anteriorposterior chest diameters at the point of maximum severity, Haller found that all operated patients had a HI of at least 3.25. This concept of a HI cutoff value capable of identifying PE patients who required treatment was widely adopted. Since its inception, the HI has been used as a biomarker for classifying severity of PE for both clinical and research purposes. Most health insurance companies that cover expenses for surgical repair of PE require patients to have a HI of 3.25 or higher. Despite its widespread use in clinical practice and research, a 3.25 cutoff has never been validated.
One major challenge in referring to the HI as a standard severity measurement, and subsequently using it for medical decision making, is that HI is not consistently defined. Among medical practitioners, there is considerable variability in HI measurement techniques [2]. Points of contention include the axial level used to measure the anterior-posterior chest depth (i.e., defined by the deepest level of the severity versus a standardized anatomical landmark), the respiratory phase at which the measurement is acquired, and the proper imaging modality to use. Even subtle changes in measurement technique can lead to inclusion or exclusion from treatment consideration. For instance, it is well known that during deep inspiration, just as for certain chest shapes, HI values are underestimated [3]. If PE severity is the basis for surgical candidacy, it then becomes critical to evaluate whether or not HI correlates clinically with objective and subjective severity. Objective severity is traditionally defined by the physiological impacts of PE. In this regard, multiple studies have shown little to no relationship between HI and cardiopulmonary function [4,5].
Attempts at quantifying subjective severity can usually be ascertained through symptom assessment or quality of life questionnaires. While subjective PE severity tends to increase during growth, symptoms may continue to progress after peak height is reached. Consider an 18-year-old male with PE and infrequent dyspnea on exertion. Suppose he has a HI of 3.15. Hence, he would likely be denied financial coverage for surgery on the basis of the surgery being for cosmetic reasons. Even if this patient’s dyspnea becomes more frequent at age 25, he may again be denied surgical care, given that his HI would probably still be about 3.15. This is not an unlikely scenario, and it remains problematic for a HIbased surgical candidacy criteria. Aside from physical symptoms, previous research has also confirmed that HI does not predict aesthetic dissatisfaction [6]. These findings, and several others, are sparking controversy over the utility of HI. In a recent survey given to 334 chest wall malformation experts, more than half considered a cutoff value of 3.25 to be incorrect [6].
Our view is that health insurance companies are lagging behind clinicians who are collectively beginning to shy away from HI. In fact, the most recent criteria recommended by donald Nuss [7], for whom the minimally invasive surgical repair of PE is named after, includes 5 distinct items (Table 1). Although a HI cutoff of 3.25 is included, it is not required. Research into many new PE-severity measurements is currently underway. Categorically, these include various sternal indexes (e.g., sternal rotation index), severity indexes (e.g., correction index), deformity indexes (e.g., asymmetry index), and cardiac indexes (e.g., cardiac compression index) [6,7]. We hope that some of these may be more correlated with objective and subjective PE severity.
Table 1: Recommended criteria for Pectus Excavatum repair.
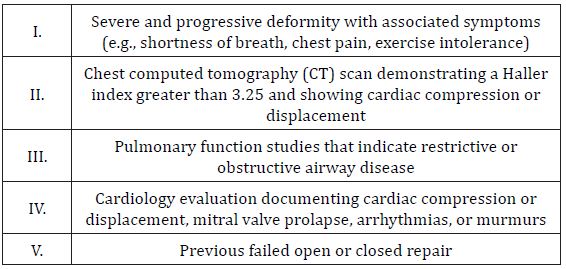
Patients should fulfill at least 2 of 5 criteria.
A HI cutoff value of 3.25 should not solely stratify PE patients into treated and untreated subgroups. This measure alone cannot explain or predict the wide spectrum of psychologic and physiologic symptoms that PE patients attribute to their deformity. Consistent with the beliefs of over half of chest wall malformation experts, we do not prefer to employ this cutoff in practice and often do not measure a HI at all. More studies are needed to identify better biomarkers of PE severity. In the meantime, health insurance companies should willingly consider factors outside of HI to enable PE patients the care they need.


