Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Arben Haziri1, Fatmir Faiku1, Arben Mehmeti2, Kemajl Kurteshi1, Imer Haziri2, Ibrahim Rudhani3*
Received: January 27, 2018; Published: February 09, 2018
Corresponding author: Ibrahim Rudhani, Medicinal Faculty, university of Prishtina, 10000 Prishtina, Kosovo
DOI: 10.26717/BJSTR.2018.02.000747
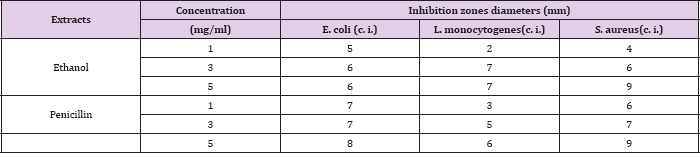
In this study the antibacterial efficiency of ethanol extract from Salvia officinalis (L.) growing wild in Kosovo were examined. Ethanol extract was tested against two gram positive bacteria Staphylococus aureus (clinical isolate), Listeria monocytogenes (clinical isolate) and one gram negative bacteria Escherichia coli (clinical isolate). The antibacterial activity was determined by using agar disc diffusion method. The inhibition zone of extract was compared to that of penicillin G. The ethanol extract showed activity in all of the concentrations 1, 3 and 5 mg/ mL towards E. coli, S. aureus and L. Monocytogenes. Ethanol extract of the plant with concentration 5 mg/mL showed a stronger antibacterial activity towards bacteria S. aureus with inhibition zone of 9 mm. The antibacterial activity of the S. officinalis (L.) was due to the presence of various secondary metabolites such as phenols and flavonoids. Hence, this plant can be used to discover bioactive natural products that may serve as leads in the development of new pharmaceuticals.
Keywords: Salvia officinalis (L.); Antibacterial activity; Agar disk diffusion method; Ethanol extract
The heterogeneous relief of Kosovo with the diversity of soil types, are responsible for the heterogeneousness ofthe Kosovo flora [1]. The secondary metabolism of the plants which are responsible for the biological activities are terpenes, phenolics and nitrogen- containing compounds. The antibiotics drugs obtained from plants are of greater interest in comparison to synthetic ones. The use of natural antibiotics from plants does not induce the secondary health effects (abdominal pain, diarrhea, cramping, headache, nausea and vomiting etc). Plant products may be used as antibiotic alternatives and do not cause resistance in bacteria [2].
The genus Salvia, commonly called sage, is the largest member of Lamiaceae or mint family containing over 900 species throughout the world [3]. Salvia officinalis L., probably the most known species of the Salvia genus and commonly known as garden or red sage, is a perennial hardy sub-shrub native to Mediterranean regions and is one of the most popular medicinal and culinary herbs used in the world [4,5]. Sage water extracts has been traditionally used for the treatment of digestive and circulation disturbances, bronchitis, cough, asthma, angina, mouth and throat inflammations, depression, excessive sweating, skin diseases, and many other diseases. In addition, they possess a number of biological activities including antiseptic, antibacterial, antioxidant, antiviral and antimycobacterial activities [6-19]. The antibacterial effect of the ethanol extracts, obtained from the medicinal plants, has been studied by a number of researchers in different parts of the world. Our research group was interested to analyze the biological activities of different medicinal plants extracts growing in different region of Kosovo. The aim of this study was to investigate the antibacterial activity of ethanol extracts of Salvia officinalis L. growing wild in Kosovo.
The S. officinalis L. plant was collected in 2015 in Kosovo. Voucher specimens were deposited in the herbarium of the Department of Plant Protection, University of Prishtina. The aereal part of this medicinal plant were air dried at room temperature within three weeks.
Preparation of Plant Organic ExtractsThe aerial part of S. officinalis (L.) was air-dried and then milled with a mixer. A piece of finely powdered material (200 g) was extracted with 70% ethanol (CH3CH2OH, 4 L) during a 24 h period (three times). After removing the solvent by vacuum rotary evaporator (EYELA N-1000, Japan) the crud product was dissolved in DMSO to prepare the solution with the concentration 1, 3 and 5 mg/mL. The solutions are used in subsequent experiments for testing their antibacterial. Etanol (analytical grade) for extraction were obtained from Sigma-Aldrich.
Antibacterial ActivityThe antibacterial activity of the S. officinalis (L.) extracts obtained by using the ethanol organic solvents are determined applying the Kirby-Bayer [19] method or disk method (d = 5.5 mm, maximum capacity 10 mg). S. officinalis (L.) extracts were tested in an in vitro experiment against bacterial strains; S. aureus (clinical isolate with code 3319), L. monocytogenes (clinical isolate with code 2653) and E. coli (clinical isolate with code 2813). For the research we used three diferent concentration of ethanol extract, 1, 3 and 5 mg/mL in DMSO as solvent and then placed in a Petri dishes (d=15 cm). The disks were incubated at 37 0C for 48 h; the control was also maintained with penicillin G dissolved in DMSO in a similar manner.
During the antibacterial activity, we have tested the extracts from the plant S. officinalis (L.) against bacteria: S. aureus (positive Gram bacteria), L. monocytogenes (positive Gram bacteria) and E. coli (negative Gram bacteria-clinical isolate). Antibacterial activity of the ethanol extracts were compared with Penicillin G- a substance, which can be found in the market and have been used with concentration 1, 3 and 5 mg/ mL as a standard. The comparison is done in accordance to the measurements of the diameter of the inhibition zone that is formed. These results are shown in Table 1.
Extracts of ethanol (1, 3 and 5 mg/ mL) shows antibacterial activities against S. aureus, L. monocytogenes and E. coli (Table 1 and Figure 1). The extracts of ethanol resulted in a lower activity than the penicillin G of the same concentration against E. coli (Table 1 and Figure 1). The extract of ethanol with concentration of 3 mg/ mL and 5 mg/mL has higher inhibition zone (7 mm) as the standard (6 mm) against L. monocytogenese. The extract with concentration 1 mg/mL resulted in lower activity than the penicillin G with the same concentration against L. monocytogenese (Table 1 and Figure 1). The extracts of ethanol with concentration of 5 mg/mL have the same inhibition zone as the standard (inhibition zone 6 mm) against S. aureus (Table 1 and Figure 1).
Figure 1: Remobilization of pseudo aneurysm's feeder artery.

Table 1: Inhibition zones diameters of S. officinalis (L.) ethanol extracts.
S. officinalis (L.) is a medicinal plant used in folk medicine for different health illnes. The aim of this research was to analyse the antibacterial activities of ethanol extracts from S. officinalis (L.) plant growing wild in Kosovo. Based on the results, the three concentrations of the extract showed a very good activity by inhibiting the growth of all the tested bacteria. Ethanol extract of the plant with concentration 5 mg/mL showed a stronger antibacterial activity towards bacteria S. aureus, E. coli and L.monocytogenes (clinical isolate). Extract with concentration 5 mg/mL showed a higher antibacterial activity against bacteria L.monocytogenes. Results obtained from ethanol extracts of S. officinalis(L.) for antibacterial activity are logical, based on numerous of studies where these extracts were analyzed in the content of flavonoids, phenols, terpenes, alkaloids, etc., and these components are responsible for the biological activity.


