Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Nora M. Al-aboud*
Received: February 02, 2018; Published: February 15, 2018
Corresponding author: Nora M. Al-aboud, Dermatology Department, King Faisal Hospital, Taif, Saudi Arabia
DOI: 10.26717/BJSTR.2018.02.000764
Raisins' natural resistance to spoilage and ease of storage and transport only serve to strengthen their appeal and widespread consumption. This study discussed the effect of taking 8gm of Raisins (Vitis vinifera L.) for 20 days on the blood samples off male volunteers where we recorded the haemoglobin levels before and after the study. The results showed up mild increment in haemoglobin readings, decrease in TIBC, increase in ferritin and decrease in transferrin. Also, there was mild increment in serum iron levels after taking Raisins. Regarding MCV, It showed up mild increament in five volunteers only. On these bases several findings support the suitability of Raisins as a source of healthy compounds for human diet, but limits in the data published till now clearly support the need of new specifically designed trials.
Keywords: Raisins; Vitis vinifera L; Dietary iron content; Iron Deficiency Anaemia
Iron is an important essential mineral which is vital for many of the biological functions of the body. Iron in the body is primarily involved in oxygen transport as well as in various biological activities like cellular proliferation, electron transfer and enzymatic reactions. Report by the World Health Organization shows that of the nutritional disorders, iron deficiency anaemia is one of the most common which has major effect on both health and economy [1]. Iron deficiency (ID) affects a major part of the population in almost every country in the world. Anaemia is also the reason for disability as well as mortality among large group of population around the globe. The susceptibility of anaemia is more in children, adolescents and women of child-bearing age including pregnant women [2].
The main cause of anaemia is not only due to low iron intake but also poor iron absorption. Anaemia is characterized by low concentration of haemoglobin and hence their tissues get less oxygen than the required amount. Anaemia results in decrease in overall physical growth of children decrease in cognitive performance as well as compromised immune status [3]. This global health problem can be addressed by improving the dietary iron bioavailability. Epidemiological studies have shown a consistent, inverse relationship between a diet rich in fruit and vegetables and a lower risk for many chronic diseases including cancer [4,5] World Health Organization. Steinmetz and Potter [6] heart disease [7,8] and stroke [9]. Researchers are also investigating the beneficial role of fruits and vegetables in inflammatory diseases [10] such as arthritis; in lowering the incidence of obesity and controlling diabetes [11,12] and in age-associated neurological problems such as Alzheimer's and Parkinson's Disease [13,14]. Fruits and vegetables also appear to have a role in the prevention of cataracts and macular degeneration [15], and may also protect from osteoporosis [16]. Finally, they may enhance the immune system and potentially modulate certain aspects of immune function. The protective effect of an abundance of fruits and vegetables in the diet is long lasting. Higher intake of fruits [17] and vegetables [18] during childhood is associated with a lower incidence of cancer and stroke, respectively, during adulthood. Raisins which is available world-wide, should be of particular interest in these investigations due to their unique phytochemical composition and the natural qualities that make raisins an appealing source of nutrients. In the United Kingdom, Ireland, New Zealand, and Australia, the word "raisin" is reserved for the dark-coloured dried large grape.
Raisins, like other fruits, are devoid of fat, saturated fat and cholesterol. They provide both soluble and insoluble fibre at levels that represent a meaningful contribution to daily fibre intake and at levels that benefit cardiovascular health. The aim of the present study is to show up the impact of Raisins (Vitis vinifera L.) intake on the level of some blood tests in a group of female volunteers.
Iron deficiency anaemia is one of the most common of the non communicable disease which needs urgent effective corrective measures. It has been reported as the major health challenge that the world is facing [19]. It is the one of the most common nutrient deficiency which is prevalent across the globe [20]. The cognitive performance as well as physical growth of children is greatly influenced by the iron content in the food they consume [21,22]. People with iron deficiency have impaired gastrointestinal functions and altered metabolism and hormone production. It has been shown in experimental animals that iron plays a key role in brain function. Anaemia is multi-factorial in nature and correcting anaemia requires an integrated approach. One of the best strategies to prevent iron deficiency is food-based approaches to increase iron intake through consumption of food rich in iron. Food based strategies not only help prevent anaemia but also bring along other long-term nutritional benefits.
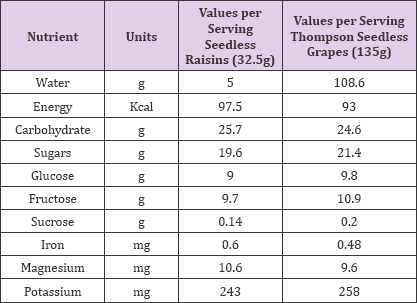
Raisins, like most fruits, possess a combination of an appealing, sweet taste and nutritional value. Raisins provide essential nutrients, soluble and insoluble fibre, and health protective bioactive components, or phytochemicals. Table 1 shows selected nutrient compositions of raisins and of Thompson Seedless grapes as a reference [23]. Both raisins and grapes provide similar amounts of sugar (19.6g and 21.4g, respectively), divided almost equally between fructose and glucose with minimal amounts of sucrose. Raisins, like all fruits, are high in potassium and low in sodium. Compared to other fruits, they are high in magnesium and iron.
Table 1: Selected Essential Nutrients in Grapes and Seedless Raisins (USDA,2007).
Raisins are among the 50 major food contributors of boron in the American diet, having the highest concentration of boron at 2.2mg per 100g [24] . Boron, a putative essential trace element is crucial for the growth and maintenance of healthy bones and joints [25,26]. Controlled animal studies have shown that boron is essential for normal growth of both bone and cartilage and appears to have a role in the maturation of the bone growth plate [27]. Boron supplementation in rats and chicks has been shown to increase bone strength [28]. Boron may also have a preventive or therapeutic effect on osteoporosis by reducing bone calcium loss in postmenopausal women. Controlled boron deprivation studies indicate that boron has an essential role in maintaining bone density. In clinical studies including both men and women, boron supplementation after consumption of a low-boron diet increased previously suppressed 25-hydroxycholecalcipherol (vitamin D) levels. Supplementation of a low-boron diet with an amount of boron commonly found in diets high in fruits and vegetables induced changes in postmenopausal women consistent with the prevention of calcium loss and bone demineralization [29].
Raisins are a good source of soluble and insoluble fibre and help meet dietary fibre recommendations. The total dietary fiber content of raisins is 3.7g/100g, according to the USDA Nutrient Database [30]. However, other investigators have reported higher fibre levels for sun-dried, dipped and golden raisins: 5.05g 100g, 5.37g/100g and 5.05g/100g, respectively [31]. Prebiotics in raisins may play a role in colorectal cancer protection. Research using experimental animal models indicates that fructans have anti carcinogenic properties. For example, in rats, dietary fructans inhibit the formation of chemically-induced aberrant crypt foci. These are neoplastic lesions in the colon from which adenomas and carcinoma may develop [32-34]. The growth of bifida bacteria, have been shown to modify ras ocogene activity. Ras-gene activation is one of the earliest and most frequent genetic alterations associated with human cancers, specifically with colon cancer. Elevated levels of ras-p21 (the ras gene product) have been correlated with increased cell proliferation. Studies in rats have shown that Bifidobacteria longum can significantly suppress the expression of ras-p21 in the colonic mucosa and reduce tumour incidence [34]. In studies using human cell lines, fructan fermentation products have been shown to inhibit tumour cell growth, modulate differentiation and reduce metastatic activities [35].
Prebiotics in raisins may offer cardiovascular benefits through a triglyceride- and cholesterol-lowering effect. A recent metaanalysis of 16 clinical studies [23,36] showed that dietary fructans significantly reduce serum triglycerides. The mechanism for this effect is still not clear, but it appears that fructans, like other soluble dietary fibres, reduce the capacity of hepatocytes to synthesize triglycerides from palmitate [37] and so lower net hepatic triglyceride synthesis. Given that fructans are not absorbed, how this effect is mediated is still a matter of speculation. One possible mechanism involves its fermentation products: an increased production of SCFA in the large intestine, particularly of propionic acid. This fatty acid has been shown to inhibit lipogenesis in isolated hepatocytes [38]. Other mechanisms involve modification of intestinal synthesis of cytokines and incretins, which enhance postprandial insulin secretion and so affect hepatic lipogenesis.
A recent study [39] found that eating raisins daily raises serum ORAC in healthy but overweight individuals. ORAC measures the combined capacity of antioxidants, particularly water soluble ones, to lower levels of oxidants that may damage susceptible molecules. ORAC is thus influenced by blood levels of vitamin C, uric acid and flavonoids. In this study, 17 overweight men and women either ate 90g raisins or isocaloric placebos for 14 days in a randomized, cross-over design while following a low-flavonoid diet. After the raisin intervention, individuals had higher blood ORAC levels. This suggests that the antioxidants in raisins, probably phenolic compounds and flavonoids, may raise the antioxidant defense capacity of blood either through direct scavenging or by modulating the activity of other antioxidants. It has been hypothesized that fruit consumption may also raise total blood antioxidant capacity because of the higher uric acid levels resulting from fructose metabolism.
Raisins as part of a diet high in unrefined foods have been shown to have a beneficial effect on blood lipid levels. Bruce et al. [40] showed that a diet rich in unrefined foods, that also provided 126g of raisins daily, lowered total cholesterol and LDL cholesterol by 13% and 16%, respectively, in hyperlipidemic volunteers. In a randomized study, Gardner et al., showed that a plant-based, low-fat diet (which included raisins as snacks) significantly lowered total and LDL cholesterol levels among moderately hypercholesterolemic volunteers compared to those who consumed a more conventional, low-fat diet based on convenience foods. While many foods can account for the observed hypolipidemic effect of the experimental diets, these findings show that sun-dried raisins can be consumed as part of a cholesterol-lowering, plant-based diet.
Epidemiological studies have consistently shown that diets rich in fruits and vegetables help prevent many types of cancer. The inverse association between fruit and vegetable consumption and cancer incidence is strikingly consistent, and has led organizations around the world to recommend that populations increase their daily intake of these foods. Many components found in fruits and vegetables have been proposed as candidates for the observed protective effects, such as soluble and insoluble fiber and, more recently, polyphenolic compounds, particularly flavonoids. Raisins are an important dietary source of both of these cancer-protective compounds. Health benefits of fiber in raisins, including its potential cancer protective effect.
Flavonoids are potent antioxidants in vitro and are able to scavenge a wide variety of reactive molecules that can harm cell constituents. They have been shown to repair DNA damage; modulate nuclear receptors and gene expression; stimulate or inhibit enzymes that detoxify or activate carcinogens [41], and influence the cell cycle, signaling pathways [42] and angiogenesis [43]. The main flavonoids present in raisins are the flavonols quercetin and kaempferol. Quercetin inhibits carcinogen-induced cancer in many animal models [44-46]. In cell culture studies, quercetin suppresses the growth of ovarian, prostate and breast cancer cells and inhibits proliferation of ovarian and lung tumour cell lines [47].
Diets high in fruits and vegetables have been associated with a lower risk of developing cancer and other chronic diseases. Over the years, many phytochemicals, bioactive compounds that contribute to these benefits, have been identified and studied. Scientists believe that it is the additive and synergistic effect of phytochemicals in fruits and vegetables that are responsible for their anticancer activity [48,49]. The benefit of a diet rich in fruits and vegetables is a result of the complex mixture of phytochemicals present in them. Raisins, with their unique combination of nutrients, polyphenols and fiber are an important ingredient of a dietary strategy for optimal health.
a. Plant: Raisins (Vitis vinifera L.) was purchased from a local market, makkah, Saudi Arabia.
b. Subject: Seven apparently healthy female volunteers (age range, 22 to 24 years) received 20 days of oral dried raisins, 8gm of dried raisins was given to each in the morning.
c. Study design: The iron status of the subjects was assessed at onset of the study (sample A) by assaying a venous blood sample for haemoglobin, total iron binding capacity ,serum ferritin, serum transferrin, Mean corpuscular volume and serum iron . Similar tests were also performed after the discontinuation of supplementation (sample B). Blood samples were taken at private laboratory in the city of Makkah. Blood samples were withdrawn from every volunteer and placed in test tubes which contain anticoagulant substance. First test tube was utilized to do complete blood count by UDI hemolysis machine, and the second test tube was placed in central centrifugation to isolate the plasma and then to analyze ferritin, transferrin and total iron binding capacity separately by MINE Vidas machine. An iron level in serum was analyzed by CUBAS C3.
Table 2 is showing the result of laboratory investigations for Haemoglobin (Hb), Total Iron Binding Capacity(TIBC), Ferritin (Fe), Transferrin (Tf), Serum iron(SI) and Mean corpuscular volume (MCV) from blood samples of the subjects to be tested before the experiment and after 20 days of taking 8 gm of Raisins in a daily basis, and it showed up very clearly in Table 2 and Figure 1 that there is a mild increment in Hb levels of the subjects after taking Raisins and the increment percentage was ranging between 2% and 6. 2%, Haemoglobin is the most widely used indicator to measure anaemia at the population level since it is quick and easy to use in the field. However, it reflects only severe forms of anaemia since it does not capture iron stores and does not allow for differentiation between anaemia and IDA, on the other hand the levels of TIBC were decreased for all subjects after taking Raisins in relation to pre-test levels, and the decrement percentage was ranging between 0.2% and 16.7% as in Table 2 and Figure 2, this decline in TIBC may be related to improvement of iron store as a result of Raisins intake.
Figure 1: Hemoglobin (Hb) values before and after Raisins (Vitis vinifera L.) supplementation.
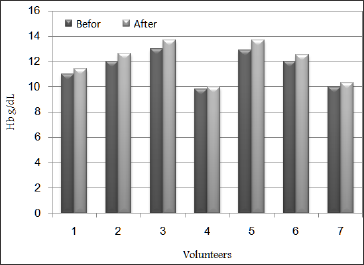
Table 2: Haemoglobin (Hb), Total Iron Binding Capacity (TIBC), Ferritin (Fe), Transferrin (Tf), Serum iron(SI) and Mean corpuscular volume (MCV) values before and after Raisins (Vitis vinifera L.) supplementation.
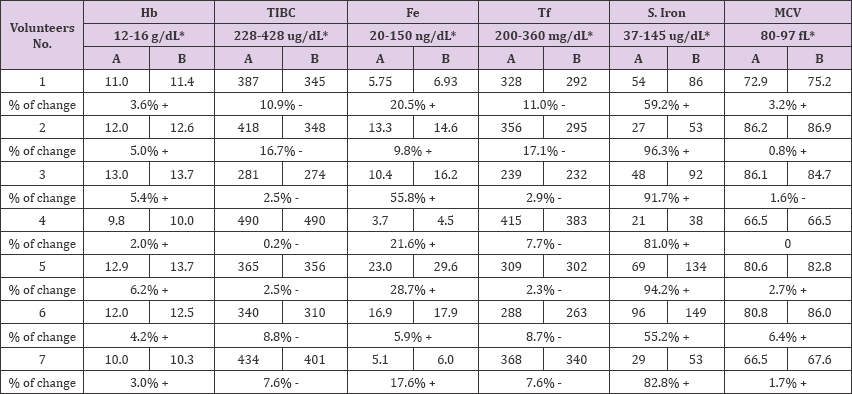
Figure 2: Total Iron Binding Capacity (TIBC) values before and after Raisins (Vitis vinifera L.) supplementation.
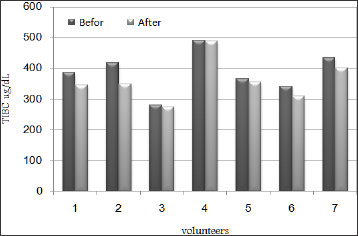
Figure 3: Ferritin (Fe) values before and after Raisins (Vitis vinifera L.) supplementation.
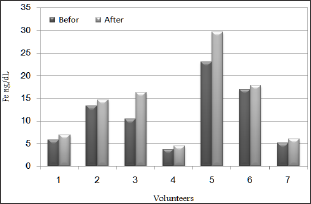
Figure 4: Transferrin (Tf) values before and after Raisins (Vitis vinifera L.) supplementation.

Figure 5: Serum iron (SI) values before and after Raisins (Vitis vinifera L.) supplementation.
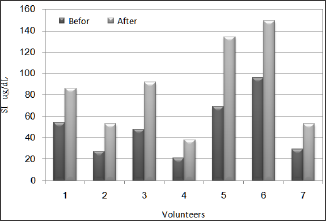
Figure 6: Mean corpuscular volume (MCV) values before and after Raisins (Vitis vinifera L.) supplementation.
Ferritin levels for all subjects showed up mild increment after 20 days of taking Raisins in relations to the levels that have been recorded before the test and the increment percentage was ranging between 5.9% and 55.8% as in Table 2 and Figure 3. Serum ferritin is considered the best indicator of IDA since it reflects iron stores and so detects early stages of deficiency. Under steady state conditions, serum ferritin concentrations correlate well with total body iron stores. Thus, serum ferritin is the most convenient laboratory test to estimate iron stores [50-52]. Ferritin has normally been considered as a storage compound from which iron is readily mobilized either into the transferrin-bound plasma pool or for intracellular haem synthesis, so this increase in Fe may be related to improvement of iron store as a result of Raisins intake. On the other hand, Tf levels were decreased in blood samples of the subjects after 20 days of taking Raisins in comparison to the levels that have been recorded before the test as in Table 2 and Figure 4 and the decrement percentage was ranging between 2.3% and 17.1%. The decrease in Transferrin level may be as a result of decrease for iron demand.
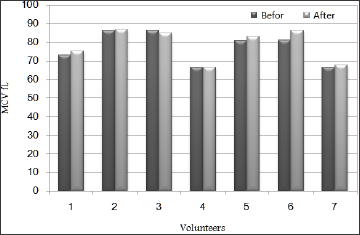
Serum iron levels at all subjects showed up enormous increment after taking Raisins and the levels recorded were 59.2%, 96.3%, 91.7%, 81%, 94.2%, 55.2% and 82.8% respectively as in Table 2 and Figure 5, This probably indicates that Raisins an excellent source of iron. Also, MCV levels showed up mild increment at five subjects after taking Raisins and the increment percentages were 3.2%, 0,8%, 2.7%, 6.4% and 1.7% respectively as it appears in Table 2 and Figure 6, this is may be related to the fact that 20 day are not sufficient to have a satisfactory results regarding MCV level and we need a bit longer duration. also it is may be explained by the length of RBC life span which takes approximately 120 day [53]. Iron deficiency anaemia is one of the most common of the non-communicable disease which needs urgent effective corrective measures. It has been reported as the major health challenge that the world is facing WHO [20]. It is the one of the most common nutrient deficiency which is prevalent across the globe.
Anaemia has a major consequence on our health and it also causes disability and decreased performance. The contribution of iron in body is mainly through diet and the bioavailability of iron depends on the source whether heme iron or non-heme iron [54]. The conventional laboratory tests of iron status, serum iron, transferrin/total iron-binding capacity (TIBC), transferrin saturation, and ferritin are widely used in clinical practice, although they are considerably influenced by acute phase responses, which complicates the clinical interpretation of the test results [55-57]. One of the best strategies to prevent iron deficiency is food based approaches to increase iron intake through consumption of food rich in iron. Food based strategies not only help prevent anaemia but also bring along other long-term nutritional benefits [58,59].
Seven apparently healthy female volunteers (age range, 22 to 24 years) received 20 days of oral dried raisins, 8gm of dried raisins was given to each in the morning. The iron status of the subjects was assessed at onset of the study by assaying a venous blood sample for haemoglobin, total iron binding capacity, serum ferritin, serum transferrin, Mean corpuscular volume and serum iron. Similar tests were also performed after the discontinuation of supplementation. The results showed up mild increament in Hb levels of the subjects after taking Raisins and the increment percentage was ranging between 2% and 6.2%. The levels of TIBC were decreased for all subjects after taking Raisins in relation to pre-test levels, and the decrement percentage was ranging between 0.2% and 16.7%.
Ferritin levels for all subjects showed up mild increment after 20 days of taking Raisins in relations to the levels that have been recorded before the test and the increment percentage was ranging between 5.9% and 55.8%. Transferrin levels were decreased in blood samples of the subjects after 20 days of taking Raisins and the decrement percentage was ranging between 2.3% and 17.1%. Serum iron levels at all subjects showed up enormous increment after taking Raisins and the levels recorded were 59.2%, 96.3%, 91.7%, 81%, 94.2%, 55.2% and 82.8% respectively. MCV levels showed up mild increment at five subjects after taking Raisins and the increment percentages were 3.2%, 0,8%, 2.7%, 6.4% and 1.7% respectively.
In conclusion, we can summarize four principle strategies for correcting iron deficiency, either alone or in combination. These are education combined with dietary modification, to improve iron intake and bioavailability, iron fortification of foods and the new approach of biofortification strategies include plant breeding and genetic engineering. Finally the inclusion of raisins in a well balanced diet can contribute in promoting human health.


