Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Yehia Abdel-Galele Mahmoud*1,2, Fawy M Abd El Latif3, Amr A Amra3 and Osman A Osman3
Received: February 20, 2018; Published: March 08, 2018
*Corresponding author: Yehia Abdel-Galele Mahmoud, Albaha University, Department of Biology, Al Aqiql, Saudi Arabia
DOI: 10.26717/BJSTR.2018.02.000828
Several new heterocyclic derivatives containing fused pyrazoloxazine moieties were synthesized via cycloaddition reactions of bi- nucleophilic dentates, including hydrazine hydrate and its derivatives as well as active methylene compounds and aniline derivatives, resulting in novel five-, six- and seven-membered heterocyclic compounds. Due to increased microbial resistance to currently available drugs accentuate a real problem, so novel antimicrobial drugs are needed. Pyrazoles are good candidate as antimicrobial agents, where they have low-molecular- weight compounds, nonvolatile and chemically stable. The novel synthesized five-, six- and seven-membered heterocyclic compounds were tested for their antimicrobial activity against different pathogenic microorganisms inside Albaha area. Bacterial species included Staphylococcus aureus, Staphylococcus epidermidis, Salmonella enteritidis, Streptococcus faecalis, Streptococcus agalactiae, Klebsiella pneumonia, Proteus mirabilis, Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterobacter aerogenes. However, the following fungi were tested: Aspergillus niger, Aspergillus fumigates, Penicillium chrysogenium, Trichophyton rubrum and Candida albicans 157 and Candida albicans causes candidiasis. Staphylococcus aureus 119 was largely inhibited were observed after contact with synthesized pyrazoloxazine derivatives 1, 5, 8, 10, 13 and 24. Of the susceptible bacteria, Klebsiella pneumonia and Enterobacter aerogens 157 were the least affected, with inhibition zones between 10 and 12 mm. The pyrazoloxazine derivatives 3, 4, 25, 2Y, Ox1 and Ox2 inhibited all the fungi tested, including Aspergillus niger, Aspergillus fumigatus, Penicillium chrysogenium and Trichophyton rubrum. The zones of inhibition ranged from 40 to 14 mm/disc for these organisms. The current study demonstrates that the newly synthesized pyrazoloxazine derivatives have a good inhibition capacity against most tested pathogenic bacterial and fungal species.
Keywords: Pyrazole-Oxazine; Five-Six and Seven-Membered Heterocycles; Microorganisms; Pathogenic Microbes; Antimicrobials; Albaha
Increased microbial resistance to currently available drugs is a major cause of morbidity and mortality throughout the world.Thus, novel antimicrobial drugs are needed. The use of heterocyclic agents is an attractive alternative. Compared to conventional antimicrobials, they are low-molecular-weight compounds that are nonvolatile and chemically stable and do not easily permeate the skin. Pyrazoles, also called as azoles, are five-membered ring heterocyclic compounds with two adjacent nitrogen atoms [1].Recently, pyrazole derivatives have been found in nature [1]. For example, β-[1-pyrazolyl] alanine was isolated from watermelon seeds (Citurllus lanatus). The most common use for pyrazoles is to treat inflammation and inflammation-associated disorders such as arthritis [2]. Pyrazole derivatives are the subject of many studies due to their potential antimicrobial [3], Antiviral [4],Antitumor [5,6], Antihistaminic [7], Antidepressant [8], Insecticidal and fungicidal properties [9]. Pyrazole derivatives function as 5-a-reductase inhibitors [10]. In addition to having antiproliferative [11], Antiparasitic [12] and herbicidal properties [13]. Several pyrazoles have also been reported to have anti-inflammatory [14] and antiprotozoal effects [15,16].
Which render them valuable as medicinal and plant-protecting agents? Furthermore, the current literature indicates that 1; 2-pyrazole derivatives possess various biological activities [17]. Recently, acyclonucleosides, which are heterocyclic compounds bound to an acyclic sugar moiety, have gained worldwide attention because of their high potential as chemotherapeutics [18,19]. Substituted pyrazole and its analogs have been used as precursors for the synthesis of various biologically active molecules. Recently,improvements in microwave chemistry have dramatically reduced reaction times, which have been demonstrated in several fields of organic chemistry [20]. Additionally, microwave-assisted organic synthesis has shown significant improvement in the generation of combinatorial small molecule libraries [21].
The goal of this study was to synthesize novel pyrazoloxazine derivatives and to determine their antimicrobial activity with the cooperation of the Blood Bank in Albaha, Saudi Arabia. We chose the most common pathogenic microorganisms (bacteria and fungi) from Albaha county, including Staphylococcus aureus (skin-wound- abscess infections, hospital-acquired pneumonia, gastroenteritis),Staphylococcus epidermidis (endocarditis, urinary tract infections, skin-wound-abscess infections), Salmonella enteritidis (gastroenteritis), Streptococcus faecalis (urinary tract infections, wound, and soft issue infections), Streptococcus agalactiae (bacterial septicemia in newborns, leading to death or longterm sequelae), Klebsiella pneumonia (urinary tract infections), Pseudomonas aeruginosa (hospital-acquired pneumonia), Proteus mirabilis (urinary tract infections) and Acinetobacter baumannii (hospital-acquired pneumonia). Enterobacter aerogenes is an opportunistic pathogen associated with nosocomial infections, including urinary tract infections (especially in catheterized patients), respiratory tract infections, and bacteremia in elderly or debilitated patients. The following fungi were tested: Aspergillus niger, Aspergillus fumigates, Penicillium chrysogenium, Trichophyton rubrum and Candida albicans 157. Aspergilli cause aspergillosis and Candida albicans causes candidiasis.
We synthesized novel heterocyclic derivatives based on a fused pyrazoloxazine moiety. This moiety was synthesized via cycloaddition reactions of bi-nucleophilic dentates (including hydrazine hydrate and its derivatives, active methylene compounds, and aniline derivatives), resulting in novel five-, six- and seven- membered heterocyclic compounds. Novel heterocyclic compounds, which are versatile starting materials, were also prepared. The following heterocyclic derivatives were obtained:
a) 4-isonitroso-3-methyl-1-phenylpyrazole-5-one (oxime),
b) 5-cyano-3-methyl-1-phenylpyrazolo[4,3-b][1,4]oxazine- 6-one (oxazine),
c) 1,6-diphenyl-5-imino- 3 - methyl py razo lo [4,3:2,3] oxazino[5,6-c]pyrazole (compound 3),
d) 5-imino-3-methyl-1-phenylpyrazolo[4,3:2,3]oxazino[5,6- c]-6-[2,4-dinitro-phenylpyrazole (compound 4),
e) 1,6-diphenyl-5-imino- 3 - methyl py razo lo [4,3:2,3] oxazino[5,6-c]oxazole (compound 5),
f) 5-amino-3-methyl-1-phenylpyrazolo[4,3:2,3] oxazino[4,5-b]pyrimidine-7-one (compound 6),
g) 6-amino-5-imino-3-methyl-1-phenylpyrazolo[4,3:2,3] oxazino[4,5-b]-pyrimidine-7-thione (compound 8),
h) 5-imino-3-methyl-1-phenylpyrazolo[4,3:2,3] oxazino[5,6-b][1,4]benzoxepine (compound 9),
i) 5-cyano-3-methyl-1-phenylpyrazolo[4,3-b][1,4]oxazino- 6-(ethylcyano-acetate) (compound 10),
j) 5-cyano-3-methyl-1-phenylpyrazolo[4,3-b][1,4]oxazino- 6-(4-nitro-iminophenyl) (compound 13),
k) 5-cyano-3-methyl-1-phenylpyrazolo[4,3-b][1,4]oxazino- 6-(4-methoxy-iminophenyl) (compound 24) and,
l) 5-cyano-3-methyl-1-phenylpyrazolo [4,3-b][1,4]oxazino- 6-imino-(1-aminohexane) (compound 25).
Details of compound synthesis and characterization are presented elsewhere. Pyrazoloxazine derivatives 1, 3, 4, 5, 6, 8, 9, 10, 13, 21, 24, 25, 2Y, Ox2, Ox1, oxime and oxazine were chosen for the evaluation of antimicrobial activity against the obtained microbial strains.
The microorganisms were previously isolated and identified in patients from the Blood Bank, Albaha from different hospitals and health centers in Albaha County. Infected patient urine samples, throat swabs, vaginal swabs, wound swabs, sputum, and breath samples were cultivated in Mueller Hinton agar (for bacteria) and Sadourad dextrose medium (for fungi). These pathogens were identified after purification using Vitek2 (Biomerieux 3584, USA). Purified organisms were grown on slants in the appropriate media and stored at 40C until further use.
The Gram positive (S. aureus 119, S. faecalis, S. agalactiae, S. enteritidis, and S. epidermidis) and Gram negative bacteria (K. pneumonia, P. aeruginosa, P. mirabilis, A. baumannii, and E. aerogenes 157) were pre-cultured overnight in nutrient broth in a rotary shaker at 37°C and then centrifuged at 10,000 rpm for 5 min. The pellet was resuspended in double distilled water, and the cell density was standardized and used to inoculate Mueller Hinton medium. Petri dishes were inoculated in triplicate, and the average zone of inhibition was calculated. The fungal inocula (A. niger, A. fumigates, P. chrysogenium, T. rubrum and C. albicans 157) were prepared from 5- to 10-day-old cultures grown on potato dextrose agar. Petri dishes were flooded with 8 to 10 mL of distilled water, and the conidia were scraped using a sterile spatula. The spore suspension used for fungal inoculation was harvested.
The antibacterial activities of the pyrazoloxazine derivatives (1, 3, 4, 5, 6, 8, 9, 10, 13, 21, 24, 25, 2Y, Ox2, Ox1, oxime and oxazine) were determined using the agar cup method [22,23]. Nutrient broth was used as a basal medium for testing bacteria. Overnight bacterial cultures were spread evenly over the surface of the solidified Mueller Hinton Petri dishes. The selected bacterial culture and the inoculated plates were percolated to form wells (4 mm), which were created in the solidified agar using a sterile borer. We used 20 mg of each compound per well. Rifampicin was used as the reference (10 μg per disc) and was added in addition to the pyrazole compounds to evaluate the efficiency of the synthesized pyrazoles. The prepared plates were incubated at room temperature (approximately 25C0) for approximately 24-48 hrs. After the incubation period, the plates were collected, and the inhibition zones were recorded in mm. Dimethyl sulfoxide (DMSO) was used as a solvent to prepare the rifampicin stock solution (5 mg in 0.5 mL). Initially, 10 ng of compound per disc was prepared.
The antifungal activities of the pyrazoloxazine derivatives (1, 3, 4, 5, 6, 8, 9, 10, 13, 21, 24, 25, 2Y, Ox2, Ox1, oxime and oxazine) were determined with the disc diffusion method [24,25]. Potato dextrose agar was used as the basal medium for testing fungi. Potato dextrose agar was prepared by dissolving, yeast extract (3 gm), peptone (10 gm), dextrose (20 gm) and agar (15 gm) into 1 liter of distilled water, pH 6.0. The media was poured into Petri dishes and allowed to solidify. The potato dextrose agar plates were inoculated with each fungal culture (10 days old) by point inoculation. The filter paper discs (5 mm diameter) were impregnated with 100 μL of each compound and were placed on the inoculated plates. DMSO Table 1: Zone of inhibition (mm) for different pyrazoloxazine derivatives was used to dissolve the compounds and completely evaporated before the discs were applied. A blank disc impregnated with DMSO was used as a negative control, and Nystatin (10 ng) was used as a positive control. Antifungal activity was determined after 72 hr incubation at 28°C. The diameters of the zones of inhibition were measured in mm.
Historically, microbial infections have been a major cause of death in humans. During the 19th century, tuberculosis, diarrhea, urinary tract infections, diphtheria and pneumonia were the main causes of human morbidity. Heterocyclic pyrazole is a well- known five-membered ring with two adjacent nitrogen atoms. This compound recently received major interest for medical applications. E. coli and S. enteritidis showed complete resistance to all of the tested synthesized pyrazoloxazine derivatives (1, 3, 4, 5, 6, 8, 9, 10, 13, 21, 24, 25, 2Y, Ox1, Ox2, oxime, and oxazine) at 20 mg per well of culture media. S. aureus, S. epidermidis, S. faecalis, S. agalactiae, K. pneumonia, P. aeruginosa, P. mirabilis, A. baumannii 116 and E. aerogenes 157 showed susceptibility or resistance to the pyrazole compounds (Table 1). Among heterocyclic compounds, pyrazoles constitute a large group with a broad spectrum of biological activity [26].
Table 1: Zone of inhibition (mm) for different pyrazoloxazine derivatives against pathogens isolated from patients inside Albaha County.
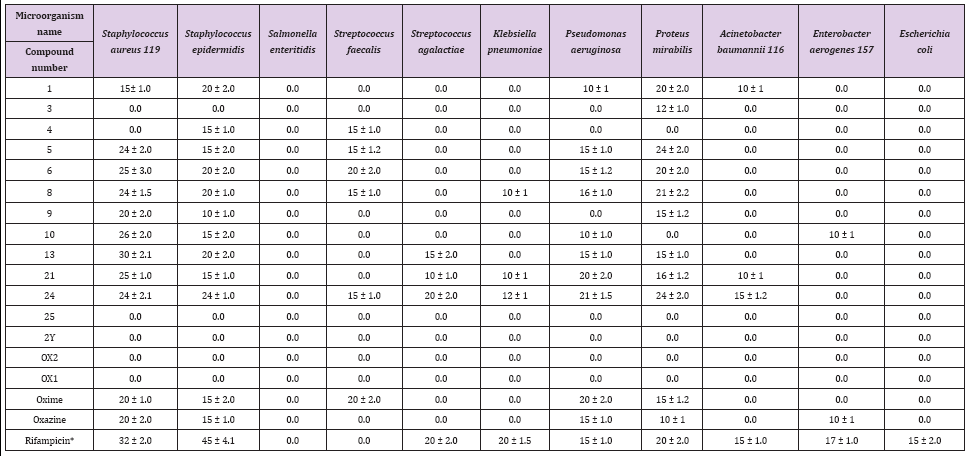
These compounds are present in nature and are constituents of nucleic acids, amino acids, alkaloids and hormones. Thus, pyrazoles may be misincorporated during synthesis of the aforementioned compounds, leading to an incorrect structure and loss of function. Additionally, pyrazoles can inhibit the enzymes responsible for its synthesis, such as nucleic acid gyrase in bacteria [27]. The maximum and minimum recorded inhibition zones were 40 and 10 mm, respectively, for all tested pyrazoles against the pathogenic bacteria species (Table 1). S. aureus 119 exhibited a 40 mm zone of inhibition after contact with the synthesized pyrazoloxazine derivatives 1, 5, 8, 10, 13 and 24. S. enteritidis and E. coli were completely resistant to all of the synthesized pyrazole derivatives. K. pneumonia and E. aerogens 157 were the least affected bacteria and exhibited zones of inhibition between 10 and 12 mm (Table 1).
Recently [28] Stated that pyrazole compounds exhibit their activity against specific targets (including bacteria), protein targets (including DNA gyrase, fatty acid synthetase, and glutamate racemase) and RNA to cause bacterial cell death, The growth inhibition pattern of different pyrazole compounds tested against P. aeruginosa were approximately equal for compounds 4, 6, 8, 10 and 24 and were the largest zones of inhibition for all of the tested compounds (Table 2). The order of effectiveness of the compounds against P. aeruginosa was (from greatest to least) 5, 6, 8, 10, 24, 9, oxime, oxazine, 1, and 4. However, compounds 3, 4, 25, 2Y, Ox2 and Ox1 had no effect on P. aeruginosa. The order of compound effectiveness against P. mirabilis was (from greatest to least): 4, 24, 1, 6, 8, 6, 13, 12, oxime, 3 and oxazine. P. mirabilis showed complete resistance to all of the other synthesized pyrazole compounds. S. agalactiae was highly resistant to the synthesized pyrazoloxazine derivatives except for three compounds (from greatest to least inhibition): 24, 13, and 21 Compound 25 was the most potent pyrazoloxazine derivative tested against S. epidermidis, which exhibited a 24 mm zone of inhibition.
Table 2: Antifungal activity of the synthesized pyrazoloxazine derivatives.
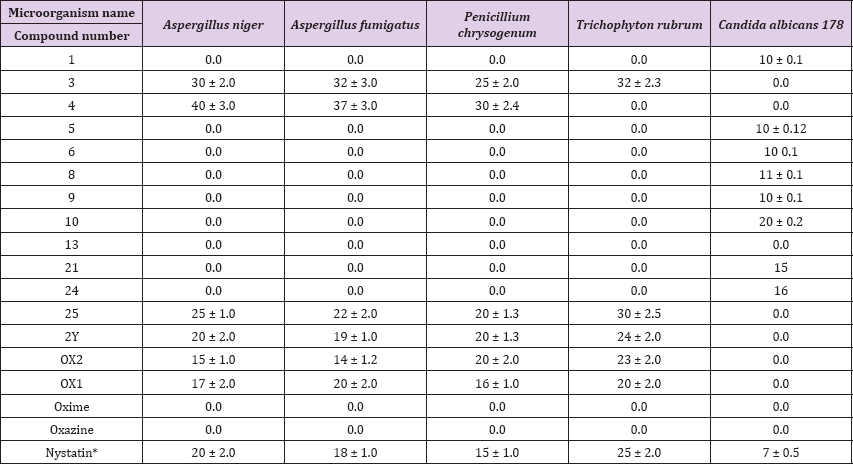
Figure 1: Antimicrobial activity of the pyrazoloxazine derivatives 24 and 25 on Staphylococcus aureus 119. andida albicans 178.

The order of effectiveness was as follows: 1, 6, 8, 13, 4, 5, 10, 21, oxime, oxazine, and 9. the other synthesized pyrazoloxazine derivatives showed no activity against S. epidermidis the inhibition pattern effectiveness of different pyrazoloxazine derivatives against A. baumanni 116 was 24, 2, and 1. The other compounds showed no activity against A. baumanni 116. The synthesized pyrazoloxazine derivatives resulted in variable zones of inhibition for S. aureus 119 (Table 1) and (Figure 1). A. Niger, A. fumigatus, P. chrysogenium and T. Rubrum were used to assess antifungal activity. Pyrazoloxazine derivatives 3, 4, 25, 2Y, Ox1 and Ox2 inhibited all of the fungi tested. The zones of inhibition ranged from 40 to 14 mm/disc. However, the pyrazoloxazine compounds 1, 5, 6, 8, 9, 10, 13, 21, 24, oxime and oxazine had no activity against fungi [29].
Stated in his study of the enzymatic basis of drug-drug interactions with systemic triazole antifungals that these compounds inhibit the enzyme in sterol biosynthesis that inhibits ergosterol synthesis (the main cytoplasmic cell membrane fungal sterol), leading to fungal death, We are interesting to decipher the mode of action of these active pyrazoloxazine compounds to increase its inhibition efficiency in near future. Where we are doing a lot of work in find out efficient antimicrobial compounds based on different strategy [30,31]. Finally, we could conclude that this study has shown that the newly synthesized pyrazoloxazine derivatives bacterial and fungal species that might be used for infections have a potent inhibition capacity against most examined pathogenic treatments (Table 3).
Table 3: Antifungal activity of the synthesized pyrazoloxazine derivatives.

This work was funded by the Deanship of Scientific Research, Albaha University, KSA. The assistance of the Deanship is gratefully acknowledged. Also, appreciation was extended to staff of microbiology in Albaha Blood bank.


