Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Michat J Markuszewski*2, Krzesimir Ciura1, Magdalena Buszewska 'Foraita2, Julia Jacyna2, Marta Kordalewska2, Michał Szczypior3, Wojciech Połom3, Joanna Nowakowska , Marcin Markuszewski3, Marcin Matuszewski3 and Roman Kaliszan2
Received: March 16, 2018; Published: April 03, 2018
*Corresponding author: Michał J Markuszewski, Department of Biopharmaceutics and Pharmacodynamics, Medical University of Gdansk, Al Gen J Hallera 107, 80-416 Gdansk, Poland
DOI: 10.26717/BJSTR.2018.03.000906
The aim of the study was to develop and optimize a simple, fast and applicable method for the quantitative determination of methylene blue. Different parameters, such as composition of separation buffer, pH value and injection time were investigated in order to obtain the best peak shape and reproducibility within the shortest analysis time. The method developed demonstrated linear response over the range of 20100 ^g/ml. Micellar electrokinetic chromatographic assay proposed is favorable in terms of the overall analysis time and method simplicity.
Keywords: Methylene Blue; Micellar Electrokinetic Chromatography; Method Development
Abbrevations: MB: Methylene Blue; CE: Capillary Electrophoresis; LC: Liquid Chromatography; MS: Mass Spectrometry; DAD: Diode Array Detection; UV-VIS: Ultraviolet-Visible Spectroscopy; SDS: Sodium Dodecyl Sulfate; BGE: Background Electrolyte; MECK: Micellar Electrokinetic Chromatography; CZE: Capillary Zone Electrophoresis; CPA: Corrected Peak Areas
Methylene blue (MB, C.I. 52015) is one of the most popular dyes from the textile industry point of view and is also widely recognized in cosmetics, printing, environmental chemistry and medicine [1].MB has a broad spectrum of pharmacological activity. Its most commonly utilized feature refers to visualization that is why MB is usually used as an indicator dye for surgical and diagnostic marking.Moreover, MB can be used to treat methemoglobinemia, cyanosis and mild urinary infections (due to its antibacterial activity) [2].Its use in the other fields needs to be carefully evaluated and supported by preliminary safety studies. Because of this fact, simple method for the determination of MB in biological samples needs to be developed and optimized. Due to physicochemical properties of MB, its determination can be provided by several analytical techniques. Among them, capillary electrophoresis (CE) and liquid chromatography (LC) coupled with mass spectrometry (MS), diode array detection (DAD) and ultraviolet-visible spectroscopy (UV- VIS) can be listed [3,4]. The main goal of the conducted study was to develop simple method providing fast determination of MB.
All experiments were performed with the use of Agilent 7100 Capillary Electrophoresis System (Agilent Technologies, Palo Alto, CA, USA) equipped with a diode array detector. Fused-silica capillary (Polymicro, Wes Yorkshire, UK) of a total length equal to 34 cm (effective length 25.5 cm) x 50 μm i.d. was applied. Using DAD, peak wavelengths of 292nm and 590 nm were selected for MB detection. Data handling and processing were computer-controlled by OpenLab CDS, Chem Station Edition for CE & CE-MS data (version 1.9; Agilent Technologies, Palo Alto, CA, USA).
Sodium hydroxide was purchased from POCH (Gliwice, Poland). Methylene blue (0.01g/ml, prescription drug) was obtained from STEROP (Brussels, Belgium). Methanol (for HPLC ≥99.9%), 2-amino-2-hydroxymethylpropane-1,3-diol (Tris; >99.8%), sodium dodecyl sulfate (SDS) and phosphoric acid were purchased from Sigma Aldrich (Steinheim, Germany). Stock solutions of phosphoric acid (500mM), TRIS (400mM) and SDS (200mM) were prepared with deionized water. Water was purified with Millipore Direct-Q 3 UV Water Purification System (Millipore Corporation, Bedford, MA, USA). Separation buffer (background electrolyte, BGE) was prepared each day by dilution of the stock solution. BGE was degassed by sonication (20 min) before the use.
The method developed was based on micellar electrokinetic chromatography (MECK). This technique was used instead of capillary zone electrophoresis (CZE) since MB is adsorbed on the capillary wall in pH above 5.4 [5]. Another advantage of the use of MECK is a very short analysis time while using inverse polarity mode, which is related to the absorption of MB to negatively-charged micelles. In this preliminary study, the addition of two organic solvents, methanol and acetonitrile, in different concentration were examined. Data were collected at a wavelength of 292nm providing the highest signal to noise ratios.
Conditioning of new capillaries was performed twice, at the beginning and at the end of every sequence run. Capillary inner wall was flushed with pure methanol (10min), 0.1 M NaOH (10min) and deionized water for further 10 min. Each day, before the first analysis, capillary was also washed for 10 minutes with BGE. Between consecutive runs, the capillary was rinsed for 30s with BGE. All conditioning steps were performed by applying 900 mbar pressure. Prepared samples were injected hydrodynamically (50mbar, 10s). Afterwards, the procedure of immersing the ends of capillary in water was applied, in order to avoid BGE crosscontamination. The separation was carried out by applying a voltage of 25.0 kV in inverse polarity. The capillary temperature was kept constant at 25 °C (± 0.1 °C). Aqueous solution of SDS (40mM), TRIS (40mM) and phosphoric acid (50mM) with the addition of 16.7 % (v/v) of methanol was chosen as a final composition of BGE, providing pH equal to 3.1.
Appropriate volumes of commercially available MB solution were mixed with 200μl of 0.9 % NaCl and filled up with pure water Table 1: Parameters of MB concentration-response calibration cu to 1ml, obtaining 7 solutions of MB. The 100μl of each acquired solution was transferred into a dark glass vial with 900μl of pure, deionized water, constituting calibration samples. Finally, the sample was vortex-mixed (30s, 3000rpm) and placed in the auto sampler. Real samples were prepared according to procedure described above i.e. by the 9-fold dilution of MB solution of unknown concentration with 200μl of 0.9 % NaCl and water.
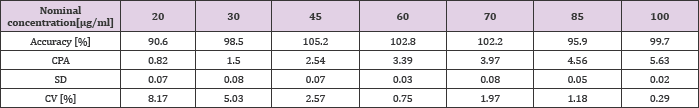
The use of methanol as an organic solvent resulted in nearly 30 % increase in peak height, compared to the values obtained for acetonitrile. Finally, 16.7 % (v/v) of methanol was added to BGE. The average corrected peak areas (CPA) (area/migration time) of MB were used for quantitative analysis. The average migration time value was 2.76 min (SD ± 0.08, CV 2.74 %). The method demonstrated linear response over concentrations ranging from 20 to 100μg/ ml (accuracy = 99.26% ± 4.88; R2 ≥ 0.994). The complete results regarding calibration curve parameters are presented in Table 1. With the use of the method developed, the amount of methylene blue at three levels (25, 50 and 75μg/ml) was successfully assessed. Exemplary electropherograms obtained during those analyses are presented in Figure 1.
Figure 1: Exemplary Electropherograms Obtained by Analysis of the MB Samples at three Concentration Levels: 25, 50 and 100 μg/ml Represented by Blue, Red and Green Lines, Respectively.
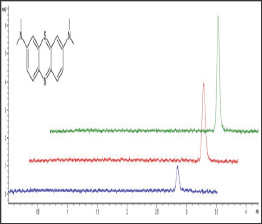
Table 1: Parameters of MB concentration-response calibration curve.
MB was tested in several studies covering the field of biomedicine to evaluate its pharmacological activity. According to literature, MB was used in methemoglobinemia treatment and urinary tract infection. Furthermore, during the last decades, the application of MB in diagnostics and surgery has been reported. One of the reasons for MB to be found an attractive dye is its fast biodegradation and toxicological safety. Therefore, we observe a need for the development of fast and simple methods for quantitative determination of methylene blue in biological samples.For instance, the method for the determination of MB in biological samples was reported by Yang. The research was focused on the determination of MB and its metabolites in blood samples with the use of capillary electrophoresis coupled with mass spectrometry.
Though, the method based on MS detection was found sensitive (determination was performed within the range of 0.3 - 15μg/ml), the analysis was quite long (MB was detected at 27.3min, while the whole single analysis lasted more than 35min) [6]. Similar study was conducted by Borwitzky's research group. MB was determined in urine samples, with the use of CE coupled with DAD. By utilizing capillary with effective length equal to 56 cm, determination of MB was obtained within 17 min, in a wide concentration range (1 - 60μg/ml). The proposed method was sensitive and provided the determination of dye at low concentration level, however the time of analysis was still relatively long [7]. Another method for the determination of MB with the use of capillary electrophoresis was reported by Hamai and Sato. However, reported method enabled the determination of MB within the analysis time about 65% longer (330s) in comparison to the method proposed in our study (200s)
The method developed provided the quantitative determination of MB in a very short time (migration time of MB = 2.76 min). Moreover, the applied analytical technique required small amount of both samples and chemical solvents. Proposed sample preparation procedure is simple, thus the optimized method is suitable for high- throughput analyses. Furthermore, the fact that calibration method does not require the use of internal standard makes the method suitable for the pharmaceutical interaction studies. Proposed method was optimized for future application in vitro studies concerning the use of MB in combination with botulinum toxin type A. Since in this diagnostic procedure, MB is used at relatively high concentration levels (50μg/ml), determination of MB was carried out within the range of 20 - 100μg/ml.


