Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
*Hanumanthachar Joshi, Charan CS and Majed Abdo Alkanad
Received: April 12, 2018; Published: April 27, 2018
Corresponding author: Hanumanthachar Joshi, Neuroresearch Laboratory, Department of Postgraduate studies and Research Sarada Vilas College of Pharmacy, KM Puram, Mysore-04, Karnataka, India
DOI: 10.26717/BJSTR.2018.04.001010
Background: Memory loss is a most disabling feature of cognitive disorders which disrupts the life styles of patients and equally affects the patient caregivers. Management and treatment of memory loss is highly challenging as no potential remedy is available at present for the complete cure. In this study, antiamnesic and neuroprotective efficacy of methanolic extract of dried leaves of Mimusops Elengi Linn. (MEE) was investigated in mice.
Methods: Elevated plus maze and Morris water maze were used for assessment of acquisition and retention. Scopolamine (0.4mg/kg, i.p.) and diazepam (1mg/kg, i.p.) induced amnesia were the exteroceptive models.
Results: MEE (100 and 200mg/kg, p.o.) significantly improved acquisition and retention in amnesic mice. MEE also exhibited reversal effects in aged mice (aging induced amnesia). MEE significantly increased acetyl cholinesterase inhibitory activity in the mouse brain. M. Elengi exhibited potential neuroprotective effects.
Conclusion: Methanolic extract of M. elengi can prove to be a potential cognition improving agent which can be beneficial in management of various cognitive disorders found in elderly.
Keywords: Acetyl Cholinesterase;Mimusops Elengi; Memory Loss; Scopolamine
In the present study, the nootropic and antiamnesic effects of methanolic extract of Mimusops Elengi (MEE) has been studies by employing both exteroceptive and interoceptive models in mice. Elevated plus maze is a neutral exteroceptive model used to assess short-term memory and Morris water maze is used to test long-term memory. Interoceptive behavioural models such as scopolamine, diazepam and natural aging induced amnesia are widely used experimental models simulating dementia in human [1]. Mimusops Elengi L. [Sapotaceae] is known as bakula in ayurveda [2]. It is a small to large evergreen tree found all over India and is cultivated in gardens as an ornamental tree and is used in the ayurvedic system of medicine for the treatment of various neurological disorders [34]. Stem bark of Mimusops Elengi possesses cardiotonic, stomachic, anthelmentic and astringent properties [5]. The bark powder along with 50g alum, 5g sodium chloride, is warmed and used for massaging on teeth in the treatment of pyorrhea by the locals [6]. The fine powder is sniffed to relieve headache, the decoction is used as a general tonic and flower in perfumery [7]. Phytochemical review of the bark of M. elengi reveals the presence of taraxerol, taraxerone, ursolic acid, betulinic acid, quercitol, lupeol [8], alkaloid isoretronecyl tiglate and mixture of triterpenoid saponins [9-10]. M. Elengi is reported to possess anti-ulcer [11] and hypertensive [12], bark improved memory in mice [13] and flowers enhanced cognition in rats [14]. The leaves are well known for analgesic, antipyretic, antioxidant and anti inflammatory properties [1517]. The present study was undertaken to evaluate the effects of methanol extract of leaves of M. Elengi on scopolamine and ageing induced amnesia in mice.
The leaves of Mimusops elengi were collected from mature trees growing in the forests of chamundi hills, Mysore, Karnataka during flowering season and identified by experts at Govt. Ayurvedic Medical College and Research Center, Mysore. The leaves were cleaned, shade dried and powdered. One kilogram of moderately powdered leaves was extracted by refluxing with methanol in soxhlet extractor for 8-10 h. The extract was evaporated to dryness under reduced pressure and temperature using rotary vacuum evaporator. The yield of dry extract from the crude powder was 16 % w/w. The methanol extract was suspended in a mixture of Tween 80: Distilled Water in a ratio of 2:8. The suspension was orally administered to animals. The volume of administration was 1ml/100 g, body weight of mice.
Scopolamine hydro bromide (Sigma Aldrich, USA), diazepam (Calmpose ®, Ranbaxy, India) and piracetam (Nootropil®, UCB India Pvt. Ltd., India) were diluted in normal saline and administeredintra peritoneally. Volume of administration was 1ml/100g. All the drugs were administered in the morning session i.e. 8 AM- 9 AM on each day. 5, 5'-dithiobis nitrobenzoic acid (DTNB, Ellman's reagent, Sigma, USA) and acetyl thiocholine (Sigma, USA) were used.
Swiss mice of either sex weighing around 18- 20 g (younger ones, aged 3-4 months) and more than 30 g (aged ones, aged 12-15 months) were used in the present study. Animals were acclimatized to the laboratory conditions for 5 days before behavioral studies. The animals had free access to food and water and were maintained under 12:12 h light and dark cycles. All experiments were carried out during day time from 0900 to 1400 h. Institutional Animals Ethics Committee (IAEC) had approved the experimental protocol and care of animals was taken as per guidelines of CPCSEA, Dept. of Animal Welfare, Govt. of India.
M. elengi methanolic extract (MEE) at different doses (102000mg/kg) was administered orally to mice with the help of a specially designed oral needle connected to a polythene tube. AR was administered at the same time on each day (i.e. 8 AM- 9 AM). During the first four hours after the drug administration, the animals were observed for gross behavioral changes if any, for 7 days. The parameters such as hyperactivity, grooming, convulsions, sedation, hypothermia and mortality were observed. The doses selected were 100 and 200 mg/kg/day.
Elevated plus-maze served as the exteroceptive behavioral model to evaluate learning and memory in mice. The procedure, technique and end point for testing learning and memory was followed as reported earlier [18-20]. The elevated plus maze for mice consisted of two open arms [16cm x 5cm] and two covered arms [16cm x 5cm x 12cm] extended from a central platform [5cm x 5cm], and the maze was elevated to a height of 25 cm from the floor. On the first day, each mouse was placed at the end of an open arm, facing away from the central platform. Transfer latency [TL] was defined as the time taken by the animal to move from the open arm into one of the covered arms with all its four legs. TL was recorded on the first day for each animal. The mouse was allowed to explore the maze for another 2 minutes and then returned to its home cage. Retention of this learned-task was examined 24 h after the first day trial.
The MWM test was employed to assess learning and memory of the animals. MWM is a swimming model where the animals learn to escape on to a hidden platform. In the present study the target quadrant was Q4. The mice were subjected to 4 consecutive trials every day with a gap of 5 minutes for 7 days continuously, during which they were permitted to escape to the hidden platform and to be remained for 20 sec. If the animal was not able to find the hidden platform within 120 seconds, the mouse was gently pushed and guided to the platform and permitted to stay on the platform for further 20 seconds. Escape latency time to identify the hidden platform in Morris water maze was the index of acquisition (learning) [21]. The starting point on every day to conduct 4 acquisition trials was changed as described below and Q4 was maintained as the target quadrant in all the acquisition trials. The starting point for dropping the mice into water maze on day 1 for four consecutive acquisition trials was in the sequence Q1, Q2, Q3 Q4 and so on.
The sequence change in starting point was as follows. Day 1: Q1, Q2, Q3, Q4 Day 2: Q2, Q3, Q4, Q1 Day 3: Q3, Q4, Q1, Q2 Day 4: Q4, Q1, Q2, Q3. Mean escape latency time (ELT) was calculated for each day of the trial. On the 8th day the hidden platform was removed, each mouse was permitted to explore the pool for 120 seconds. The animal was made to take 4 such trials with 5-minute interval time and every trial had a different starting point covering all the 4 quadrants. The mean of time spent by the animal in all 4 quadrants was recorded. The TSTQ (time spent in target quadrant) in Q4 as compared to time spent in other quadrants in locating missing platform was considered as an index of retrieval (memory). Utmost care was employed to ensure that relative location of water maze with respect to any other objects in the laboratory serving as visual clues was not disturbed during the total duration of the study. All the trials were completed between 09:00 and 17:00 hours [22].
Amnesia was induced by administration of scopolamine hydro bromide (0.4mg/kg, ip) on 8th day and the TL recorded. Retention was recorded after 24 hr. MEE (100 and 200mg/kg, po) and piracetam (200 mg/kg) were administered for 7 days successively. On 7th day, after 45 min of administration of doses, scopolamine was administered and TL was noted after 45 min. SDL was recorded on 8th day [23-25].
Diazepam, 1mg/kg, ip was administered to young mice and TL was noted after 45 min of injection on 7th day and after 24 hr. MEE (100 and 200mg/kg, po) and piracetam (200mg/kg, i.p.) were administered for 7 successive days. After 60 min of administration of the last dose on 7th day, diazepam (1mg/kg, ip) was administered. TL was noted after 45 min of administration of diazepam and after 24 hr. SDL was recorded on 8th day [26-27].
The time frame of cholinesterase activity estimation was similar to behavioral tests i.e. 8 AM- 11 AM on each day. On the 8th day the animals were euthanized by cervical dislocation carefully to avoid any injuries to the tissue. The whole brain AChE activity was measured spectroscopically using the Ellman method [28].
All the results were expressed as mean + Standard error. The data was analyzed using ANOVA followed by Tukey-kramer test.
No mortality was observed following oral administration of MEE even with the highest dose [2000 mg/kg]. MEE had no toxic effect on the normal behavior of the mice. However doses more than 1500 mg/kg exhibited profuse watery stools.
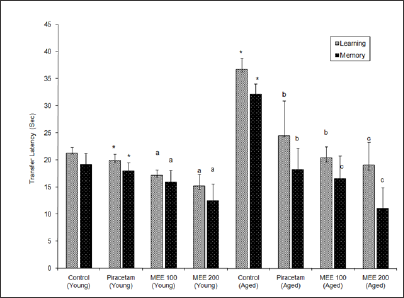
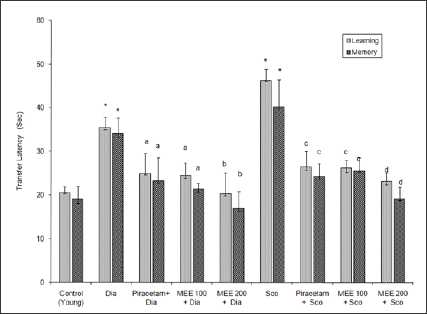
Aged mice showed higher transfer latency (TL) values on first day and on second day (after 24 hr) as compared to young mice, indicating impairment in learning and memory (i.e. ageing- induced amnesia). Piracetam (200mg/kg, ip) pre-treatment for 7 days decreased transfer latency on 7th day and after 24 hr, i.e. on 8th day as compared to distilled water treated group, indicating improvement in both learning and memory. Scopolamine (0.4mg/ kg) and diazepam (1mg/kg) increased TL significantly (P< 0.01) in young mice on first and second day as compared to control, indicating impairment of memory (Figure 1). MEE (100 mg/kg, po) decreased the TL on 7th day and 8th day in young and aged mice (P < 0.05) when compared to control groups. MEE (200mg/kg, po) more significantly enhanced the learning and memory of aged animals rather than the young mice as reflected by marked decrease in TL on 7th day and 8th day when subjected to elevated plus maze tests (Figure 1). MEE (200mg/kg, po) pretreatment for 7 days successively protected young mice (P < 0.001) against scopolamine, diazepam and ageing induced amnesia (Figure 2).
Figure 1: Effect of Mimusops elengi (MEE) on transfer latencies of young and aged mice Values are mean +S.E.M. (n=6); * indicates P< 0.01 compared to control (young) a indicates P< 0.05 compared to control ( young ); b indicates P< 0.05 as compared to control (aged); c indicates P< 0.05 compared to control (aged mice).
In MWM extreroceptive model, ME (100 and 200 mg/kg, po) exhibited significant fall in ELT as compared with control group which indicates the increase in acquisition ME (200mg/kg, po) improved TSTQ as compared to standard group indicating profound improvement in retention (memory) capacities of mice. Scopolamine (0.4mg/kg) and diazepam (1mg/kg) increased ELT significantly (P< 0.01) and decreased TSTQ, in aged mice as compared to control, indicating profound memory impairments (Table 1).
Table 1: Effect of Mimusops Elengi (MEE) on Escape Latency time (ELT) & Time Spent in TargetQuadrant (TSTQ) in aged mice.
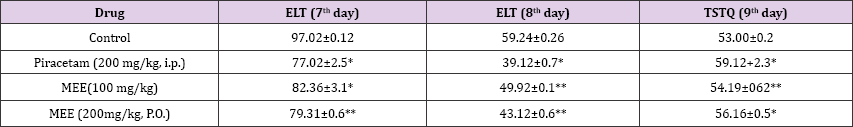
Each values represents mean+S.E.M. * P<0.01 as compared to control, **P<0.001compared to control. Oneway ANOVA followed by Tukey's post test.
The whole brain AChE activity raised significantly in mice treated with phenytoin [12mg/kg, p.o.] as compared to control and piracetam [200mg/kg, p.o.]. MEE [100 and 200mg/kg, p.o.] significantly [P<0.001] lowered AChE activity indicating profound neuroprotection and improvements in memory (Figure 2).
Figure 2:Effect of Mimusops elengi (MEE) on transfer latency of diazepam and scopolamine induced amnesic mice. Values are mean ±S.E.M. (n=6); * indicates P< 0.05 compared to control (young); a indicates P< 0.05 Compared to diazepam group; b indicates P< 0.005 compared to diazepam group. c indicates P< 0.05 compared to scopolamine group; d indicates P< 0.001 compared to scopolamine group.igure 3: Effect of Mimusops elengi (MEE) on whole brain cholinesterase (AChE) activity Values are mean ±S.E.M. (n=6); * indicates P< 0.05 compared to control (young) a indicates P< 0.05 compared to control ( young ); b indicates P< 0.05 as compared to control (aged); c indicates P< 0.05 compared to control (aged mice).
Cognitive disorders are majorly characterized by profound memory loss and inability to perform day to day normal activities which affect the life of an individual. There is sudden plunge in the incidence and prevalence of dementia across the world due to improved health benefits and increasing elderly community [29]. Despite of various advancements in science, the researchers are yet to find a radical remedy for cognitive dysfunctions. Few drugs are available such as Donepezil, rivastigmine, gallantamine but there usability for long time leads various adverse drug reactions and side effects [30]. Hence, research of phytopharmaceuticals obtained from medicinal plants of traditional origin can be beneficial [31]. In this study, the methanolic extract of leaves of Mimusops elengi improved both acquisition and retention in mice afflicted with amnesia due to normal ageing, administration of scopolamine and diazepam, which indicated that MEE has excellent potential to be used as memory restorative agent in elderly.
The authors are thankful to the Rajiv Gandhi University of Health Sciences, Karnataka, and Bangalore for the research grant. Thanks are also due to UCB India Pvt. Ltd., Vapi, Gujarat, India for gift sample of piracetam.


