Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Töysä T*
Received: April 13, 2018; Published: May 01, 2018
*Corresponding author: Töysa T, Licentiate of Medicine, Specialty General Practice, Rehabilitation Hospital Vetrea Terveys Oy, Pohjolankatu 15, FI-74100 Iisalmi, Finland
DOI: 10.26717/BJSTR.2018.04.001019
During last years the authors have discussed whether musculoskeletal diseases, as osteoathritis, could be a CHD risk factor and how to explain it. Connective tissue of the arterial wall has been suggested to participate in the progression and protection of CHD. CHD risk is composed of two risk clusters: individual and regional factors. In Finland individual major and minor risk factors have explained 40 % of CHD east-west difference. Some regional risk factors have alone or combined explained more. In this study we have benefited age standardised morbidity indices of CHD and musculoskeletal disorders (MuSk) (MuSk as an indicator of connective tissue welfare) from 2012-2014 provided by National Institute for Health and Welfare (THL) and their associations with each other, geographical factors [latitude, longitude, temperature (Temp), average annual snow cover duration [Snow.cover] - as a measure of biological activity and soil weathering], groundwater (gw) and (agricultural) soil factors [Ca, Mg, (Ca+Mg), (Mg/Ca)], gw silicon [Si.gw] and (agricultural) soil tin [Sn.soil]. Si has, as a structural component, associations with connective tissue. Sn has associations with connective tissue (glycosaminoglycan - GAG) synthesis.
Results: In general, geographic and meteorological factors explained CHD stronger than Musk or other factors. CHD and MuSk were explained significantly (p < 0.01) and similarly by factors, which have associations with connective tissue: [Si.gw] explained them [(CHD/ MuSk)] by (39/39) %, [Sn.soil] by (71/44) %, factors associated with soil weathering and biological activity: Temp by (52/39) % and [Snow. cover] by (68/49) %. MuSk alone explained CHD by 44 %.
Conclusion: Environmental factors, Si and Sn, which have associations with connective tissue structures or synthesis, explained CHD and MuSk (morbidity) significantly and similarly. MuSk explained regional CHD risk about as well as conventional factors earlier (by different age groups).
Keywords: CHD; Si; Ca; Mg, Sn; Roundwater; Soil; GAG; Collagen; Intramural friction
Abbreviations: Ca: Calsium; CHD: Coronary Heart Disease; GAG (includes e.g. Heparin; Chondroitin Sulphate and Hyaluronic Acid); GW: Ground Water; Mg: Magnesium; MuSk: Morbidity Index of Musculoskeletal Disorders or Musculoskeletal Diseases; National Institute for Health and Welfare - THL: Terveyden ja Hyvinvoinnin Laitos; Sn-tin; Snow Cover-Average annual snow cover duration (1981-2010); Soil-Agricultural Top Soil; Top Soil - Highest Soil Layer (0-25 cm); Temp - Average Annual Temperature (1981-2010).
CHD risk is composed of two risk clusters: individual and regional factors [1]. In Finland individual major and minor risk factors have explained 40 % of CHD morbidity east-west difference [2]. Some regional risk factors have alone or combined explained stronger regional CHD mortality [3]. During last years the authors have discussed whether MuSk, as osteoarthritis, could be a risk factor of CHD and how to explain this association [4]. The roles of connective tissue components in musculoskeletal welfare are generally known: collagen gives stability, elastin elasticity and glycosaminoglycans (GAG), i.e. mucopolysaccharides (including e.g. heparin, chondroitin sulphateand hyaluronic acid) moisture and lubrication [5-7]. Regeneration of arthrotic joints is possible [8]. The role of structural factors in atherosclerosis is understood by the distribution of atheromas in arterial tree: Atheromas tend to occur in arteries at points of stress [9], especially vulnerable are the coronary arteries which are exposed to stretching, compression and angulation with every heartbeat [10], pulmonary artery is as vulnerable as aorta [11], although its blood pressure (BP) is less than 1/6 to systemic BP [12].
The rupture of elastica (interna) is preceding lipid accumulation [13] simultaneously with accumulation GAG [6]. The role of GAG in atherogenesis has been disputable for more than 50 years [1315]. GAGs are thought to have a remarkable role in atherosclerosis, because of their role in fat accumulation on arterial walls [14]. Schwarz (1974) has found Si in connective tissue, in collagen, but especially in GAGs, where the Si content ranged from 37 to 1,892 ppm, median 354 [16]. He explained roles of Si: Si(OH)4 can form chains or rings with itself (-O-Si-O-Si-O-) and can combine two (to four) carbon chains (R1-O-Si-O-R2) and increase structural stability of collagen. On the other hand in the R1-O-Si-O-Si-O-R2 -complex the Si-O-Si bond is weak, can easily open and promote resilience of GAGs. Because "silicon atoms have a strong tendency to attach over oxygen atoms to each other" the ground substance can hold its architecture in spite of such slipping [16]. Intravenously and per orally administrated Si has reduced atheroma formation in rabbits by preserving elastic structures, without reducing arterial wall lipids, or ground substance (GAG) [17,5]. Inorganic perorally administred Si has increased serum HDL/LDL-ratio [18], i.e. Si can work inside the conventional risk factors.
Epidemiologic data suggest on cardioprotective effects of Si as such [3,19,20] and/or indirectly by its association with groundwater hardness and fertility of agricultural soils [3]. In the earlier mentioned Finnish study [19] Si explained better CHD than the (in those times available) individual risk factors. Tin (Sn) belongs to the 4th main group of elements as Si and carbon. Tin salts have low boiling points: stannic chloride evaporates at 114 0C, most inorganic tin derivatives below 200 oc. Good sources are "all commercial fats" [16]. Hairs are rich in tin (90 --750 ppm) [22], but which component of it is not known. A sign of Sn deficiency is baldness [23]. Hair growth is associated with the presence of chondroitin proteoglycans in the follicle environment and the cessation of hair growth is associated with their removal [24]. Excessive hair growth is one sign of mucosaccharidoses (GAG excess) [25]. Estimated tin requirement is 1.5-2.0 ppm/diet [16]. In France the estimated daily Sn intake has been ca 2.7 mg [26], in USA generally ca 0.2 - 5 mg Sn [27], but by diets including canned vegetables and fish greater than 30 mg Sn/day. The tolerable dose in humans is 2 mg/kg of body weight, i.e. ca 140 mg/d [26]. The aim of this study is to asses CHD and MuSk morbidity with geographic, gw and soil factors, in order to evaluate the risk of different factors and to see whether they could suggest on the common etiology of MuSk and CHD.
Groundwater parameters are from Groundwater database © Geological Survey of Finland 2017 [28] as in [19]. Concentrations of exchangeable (soluble) Ca and Mg from cultivated fields separately from 18 Rural Centers (RC, earlier Agricultural Advisory Centers), concerning period 1986-90 are provided by Eurofins Viljavuuspalvelu Oy [29], as in [20]. Original soluble (exchangeable) values (mg/L) are changed to mEq/L, as in [21]. Number of soil samples was 552,788. Latitude and longitude of each RC have been determined, as [21], in two-phases: first by selecting visually an approximate central commune/town of each RC in the map [30] and then via internet-search "GPS coordinates". In Lapland Rovaniemi has been selected to central city because of the general increase of agricultural soil density towards south-west. Finnish Region map with numbers and names is from Statistical Yearbook of Finland 2005 [31]. The boundaries, names and numbers of RCs and Regions deviate slightly from each other [30,31]. In Figure 1 black lines show the boundaries of Regions and the red lines the deviations made by RCs and grey lines inside them show the central communes/cities of RCs.
Table 1. Values of THL morbidity indices of CHD and Musculoskeletal disorders. Latitude, Longitude, average annual temperature and snow cover (from 1981-2010), groundwater (gw) sample number, gw and soil values of Ca, Mg, Si, (Ca+Mg) and (Mg/C a), as well as agricultural soil tin (Sn) content. Regional values of Varsinais-Suomi are calculated by weighting values of its parts by their areas [20] of cultivated arable land in 1988. The topic 'Regions' is written with commas, because a part of values are determined by RCs.
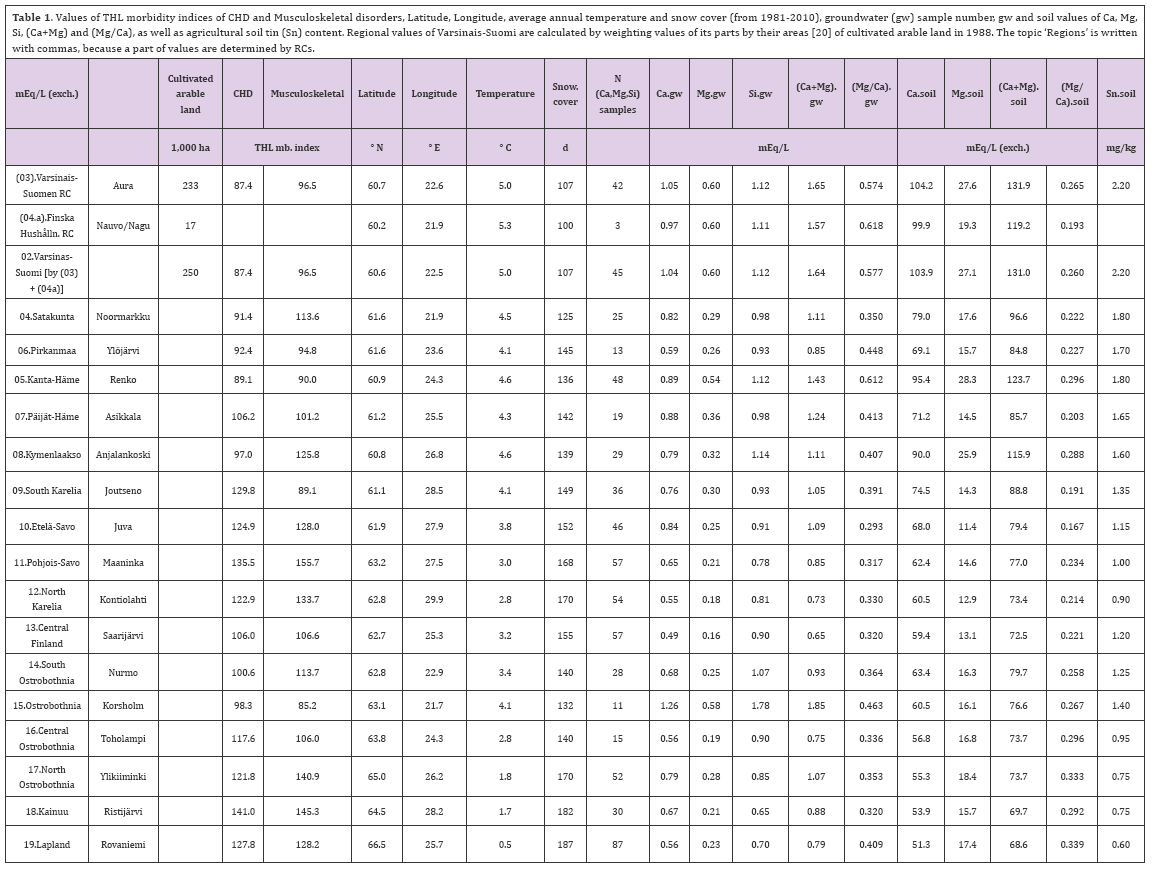
The average annual temperatures and snow cover days of RCs from 1981-2010 are visually approximated by combining FMI maps [32,33] and [30]. (Areal RC definition of temperatures is the same as in [21]. Equally by combining map of Regions and map of Geochemical Atlas concerning agricultural tin distribution [34], are the regional tin values visually approximated (Figure 2). For the values of Temp, snow cover and Sn are given sometimes slightly different values than by the central commune/city, because the density of agricultural soils is by geographic data generally growing towards south-west. Age adjusted morbidity indices of CHD and Musculoskeletal disorders are from Finnish National Institute of Health and Welfare (THL) [35]. Values of Uusimaa have been discarded, because of its nutritional dependence of the other regions and Aland , because of its scanty gw samples and suspicion that pH in the micro-milieu gives plants higher Si amounts as expected by gw values [20]. RC values of "(03). Varsinais-Suomen" and "(4b). Finska Hushallningss." are combined as in [20]. Numerical data are in Table 1 and Figure 1.
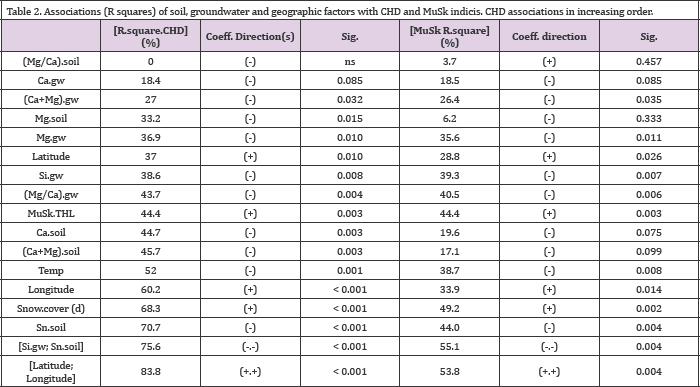
(Table 2) In general geographic and meteorological factors explained CHD stronger than Musk or other factors (Table 2) and (Figures 2&3). CHD and MuSk were explained significantly (p < 0.01) and similarly by factors, which have associations with connective tissue: [Si.gw] explained them [(CHD/MuSk)] by (39/39) %, [Sn.soil] by (71/44) %. Factors associated with soil weathering and biological activity explained them highly remarkable: Temp by (52/39) % and [Snow.cover] by (68/49) %. MuSk alone explained CHD by 44 %. Soil and gw factors explained CHD and MuSk highly similarly, with three exceptions: Mg.soil, Ca.soil and [(Ca+Mg). soil] explained CHD significantly by 33 - 46 % (p <0.01), but nonsignificantly MuSk (by 6- 20 %, p < 0.05). [(Mg/Ca).gw] explained significantly CHD and MuSk by (44/41) %, but [(Mg/Ca).soil] nonsignificantly both of them. Combined [Si.gw;Sn.soil] explained CHD and MuSk by (76/55) %. And [Latitude; Longitude] by (84/54) %(Figure 3).
Table 2. Associations (R squares) of soil, groundwater and geographic factors with CHD and MuSk indicis. CHD associations in increasing order.
In this study morbidity indicator of Musculosceletal disorders (MuSk) has been treated as synonym of morbidity of joint and bone diseases. After disclosing the (very small) group of pure muscular disorders we have a group with other disorders of spine and extremities, which can be seen as a representative of connective tissue symptoms. Even bone health is known to be associated with collagen quality, too [36]. So there is obviously rather high overlapping with MuSk and connective tissue morbidity. Repetitive intramural stretching, compression and angulation (i.e. friction) are known factors in atherosclerosis [10]. External arterial support is known to inhibit atheroma formation [37]. Angulation is obviously the extra movement, which damages the coronary arteries more than the other arteries. Benefits of Si could be associated with increased support by "high-quality" collagen and increased lubrication by "high-quality" GAGs, as in joints [8]. Rautavaara, based on the compilation of Voronkov from 1975 [38], one year after [16], explained that the main benefits of Si are explained by its being a component of elastin and increasing vascular elasticity.
Rautavaara wrote that Si increases hair growth (as Sn [23]). The benefits of Si in atherosclerosis seem to be disputable by the discovery of Nakashima: Si content is associated with the progression of atheroma formation [39]. Explanations could be: It is the question of intra-individual differences: if the primary fault is not corrected, synthesis of Si-carrying GAGs follows the chemical information and increases where it is by such information needed (older damages have for a longer time been object of such "healing efforts"). Although it is obvious that the food Si content is associated with the Si.gw, we have no data on it. Another question: is food Si increasing towards juvenile soil including carbonate soils, although there gw Si can be/may be the lowest [20], Seelig [40] has collected several findings, which are associated with the anti-atherosclerotic associations of mast cells:
a) Rats, a species resistant to atherosclerosis, have many mast cells, whereas susceptible animals have few mast cells [41].
b) Young women have more mast cells than do young men and atherosclerotic patients have fewer mast cells than do normals [41,42].
c) In breeder rats that developed the most severe spontaneous arteriosclerosis the number of myocardial mast cells was most severely depressed [43].
d) Mast cells (of these spontaneously arteriosclerotic rats) were not found in the vicinity of the arteriosclerotic lesions [43].
e) Granules of the mast cells (of these spontaneously arteriosclerotic rats) showed a marked change from metachromasia to orthochomasia, which was interpreted as indicating secretory discharge [43].
f) The decrease in numbers of mast cells in arteriosclerotic arteries has been correlated with the decrease in hyaluronic acid in arteriosclerotic arteries e.g. [44].
g) Experimental Mg deficiency has been shown to cause decreased tissue mast cells, and to increase their granulation e.g. [44].
Old textbooks wrote that mast cells are producing mainly heparin and histamine, a newer (superficial) source [5] explains that GAGs as other components of ground substance are secreted by fibroblasts. The old findings [40-45] that mast cells and their metachromatic material could possibly have benefial effect on arterial wall have possibly not been disputed. Participation of structural factors in vascular and MuSk damages is supported by the finding by Kim et al. [46,45]: nicotine - a CHD risk factor [1] - can decrease proteoglycan and collagen II synthesis (collagen II is the main collagenous component of cartilage, as old the text books wrote). There are nowadays a lot of articles concerning the harmfulness of GAGs, e.g. [14]. Possibly the new discovery of Kiran et al. (2017) that cholesterol feeding decreases GAG production and simvastatin can prevent this decrease [46] can change the status of GAG. The opposite conclusions on heparin-binding growth factor midkine by Takemoto et al. [47] vs. Kadomatsu et al. [48] and Si by Jugdaosingh et al. [49] versus Loeper [17], could be based on that [47] and [49] benefited ApoE knockout mice.
The author remembers that in Finland the tinned coffee pots were replaced by aluminous ones in the 1950's, the period of the begin of CHD epidemics [48,50] suggesting slightly on the benefits of tin, as well on the harms of aluminium (generally known antagonist of Si). Later aluminum was generally changed to stainless steel. In open non-randomized study on more than 20 patients I have seen trend-like benefits on OA patients by 1.35 mg tin daily in two weeks periods (as stannic chloride or stannous chloride). The tablets were manufactured by Helsinki University Pharmacy by order of docent Thomas Tallberg, who benefited tin (1.35 mg tablets) as some other mineral elements as supplements in his immunological cancer therapies. Estimated tin requirement is 1.5 - 2.0 ppm/diet [16]. In (Figure 2) we see that the agricultural soil Sn soil content is < 1.1 ppm in areas with highest CHD risk. Possibly some benefits of virgin oils can be explained by the low boiling point of tin, as well as differences in atherogenic potency of saturated fats could be based on their different Sn contents. Because fats contain tin [16], besides of of vitamin A and D, they are not empty calories (as sugar). Effects of tin could be mediated via GAGs. This provokes questions: are/were the side effects, e.g. hair loss, of gold and Arteparon (glycosaminoglycan polysulf.) signs of Sn (and Si) deficiency?
Concordant with earlier survey on CHD mortality [3], this regional morbidity survey did not even show significant correlation between [(Mg/Ca).soil] and CHD, opposite to time-related survey [50]. High associations of [(Mg/Ca).gw] with CHD and MuSk seem to be possible to explain by their associations with soil-type [20] and Si availability (i.e. factors promoting or inhibiting soil weathering, as [Snow.cover] - more precisely possible to calculate from (Table 1). Because of the rather good areal overlapping of the given Regions and RCs (Figure 1), the bias caused by the different data sources (by Regions or RCs) is obviously insignificant, because by Temp, [Snow.cover] and [Sn.soil] it seems not to be remarkably bigger than approximation error. Fixing Temp with available soil and gw values seems to be reasonable.
Environmental factors, Si and Sn, which have associations with connective tissue structures or synthesis, explained CHD and MuSk (morbidity) significantly and similarly. MuSk explained regional CHD risk ca equally as conventional factors earlier (by different age group).
I am very grateful to Professor Osmo Hanninen and late veterinary surgeon Seppo Haaranen for several (possibly hundreds) of discussions on these kinds of questions.


