Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Cristina Gabriela Puscasu*
Received: April 22, 2018; Published: May 01, 2018
*Corresponding author: Cristina Gabriela Puscasu, Lecturer in Periodontology Department, Faculty of Dental Medicine, Ovidius University Constanta, Romania
DOI: 10.26717/BJSTR.2018.04.001020
Background: Although factors such as diabetes, smoking, age, gender are known to act in the development and severity of periodontal disease, we aimed to investigate the association between diabetes, smoking and periodontal status in a group of eldery.
Methods: A group consisting of 205 dentate adults aged 55 years and older were selected according to their good oral hygiene, lack of other systemic conditions. Periodontal involvement was assessed according to maximum periodonta probing depth (PPD) and divided in groups: with less than 4 mm PPD, and with PPD of 4 mm or more. The diabetic condition was self-reported.
Results: A total of 44 subjects (21.5%) were active smokers (11 women and 33 men). A number of 143 (69.7%) subjects had pockets ≥4mm. Dependent relationship was found between pocket depth and the smoking status (χ2 = 3.864, df = 1, p =0.043 <α = 0.05). Calculated odds ratio (OR) showed that smokers had approximately 2.27 times higher risk of having pockets ≥4mm than non- smokers (95% CI for OR = 1.05, 5.22). No statistically significant correlations of dependence were found between diabetes and presence of pockets ≥4mm (χ2calc = 0.888, df = 1, p = 0.346 >α = 0.05).
Conclusion: Despite low proportions of smokers, tobacco use was an independent risk factor for periodontal disease in terms of deep pockets formation in this group of aged Romanian subjects.
Studies in the literature show the involvement of several risk factors in the onset, development and severity of periodontal disease. Diabetes mellitus and smoking are known as potential risk factors for periodontal disease (PD) [1]. More, studies show that a poor glycaemic control of the diabetic patient may lead to increased severity of periodontitis [2]. Previous studies [3] shown a high prevalence of smoking among adults living in Constanta, Romania, South-East Europe. Association between smoking, diabetes and periodontal status can be of interest, in the context of current periodontitis aetiology model [4].
The objective of this study was to determine if an association existed between periodontal disease, diabetes and tobacco use, in a group of older dentate individuals from Constanta, Romania.
The study population included 205 participants who were aged 55 and older (mean age 64.5±6.8), selected from patients attending dental clinics of the Faculty of Dental Medicine Constanta, Romania in 2015. The records of patients were examined to determine the patient's self-reported systemic condition (diabetes) and smoking history. In addition, periodontal probing depth (PPD) was recorded for each patient by trained, non-calibrated dentists. Maximum value obtained was considered for statistical assessment. Ethical approval was obtained from the local Ethics committee of the Faculty and each patient received information regarding the study protocol and signed an informed consent. In order to relate smoking status to severity of periodontal disease, all patients were categorized according to their maximum PPD: either with PPD of <4 mm (no site exceeding PPD of 4 mm-moderate pockets), or with PPD of ≥4 mm (deep pockets).
Subject eligibility. Subjects qualifying for the study met the following inclusion criteria:
a. 55 and over years of age;
b. More than 10 natural teeth in the mouth;
c. Regular manual or electric toothbrush users.
d. Good oral hygiene (Oral Hygiene Index below score 1)
e. Patients were excluded for one of the following conditions:
f. Antibiotic intake status within 4 weeks of the baseline examination
g. Other systemic conditions that can influence periodontal status (malignant tumors, rheumatic heart disease, heart valve prolapsed, infarct, leukemia
Statistical Analysis
The statistical analysis was performed using IBM SPSS statistics software version 20.
Descriptive statistics were performed for all patients as well as within the subgroups of smokers, non-smokers and patients categorized with periodontitis with moderate - and deep pockets. The Odds ratios (OR) and relative risk (RR) were calculated to assess the studied background factors for the occurrence and severity of periodontal disease.
Figure 1: No Statistically Significant Correlations of Dependence Were Found Between: Diabetes and Presence of Pockets.
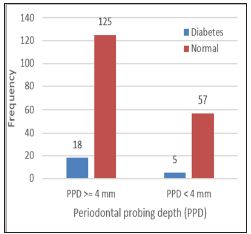
Among all 205 subjects, 44 (21.5%) were active smokers (11 women and 33 men). Overall, 143 (69.7%) subjects had pockets ≥4mm. Dependent relationship was found between pocket depth (≥4 mm/<4mm) and the smoking/non-smoking status of the subjects (χ2 = 3.864, df = 1, p =0.043 <α = 0.05) (Figure 1). The ratio of proportion of subjects with pockets ≥4mm in both smokers/nonsmokers (RR-relative risk) is 1.23, 95% CI for RR = (1.03, 1.47). Calculated odds ratio (OR) showed that smokers had approximately 2.27 times higher risk of having pockets ≥4mm than non- smokers (95% CI for OR = 1.05, 5.22). No statistically significant correlations of dependence were found between: diabetes and presence of pockets ≥4mm (χ2calc = 0.888, df = 1, p = 0.346 >α = 0.05) (Figure 2). No statistically significant correlations of dependence were found between gender and deep pockets presence (χ2calc = 1.236, df = 1, p= 0.266 >α = 0.05). The logistic regression confirmed that gender and diabetes do not act as risk factors for periodontal destruction; periodontitis is aggravated exclusively by smoking in this population (Table 1).
Using a population-based study to assess PD status in adults aged 55 years and more, we found that there is no statistical evidence that patients with diabetes are more subjected to have deep pockets, as compared to the non-diabetic ones. However, PD is a systemic disease and may also be affected by other aetiological factors, which were not considered in this study. Regarding lifestyle factors such as smoking, our findings were similar with previous studies [3-7] that smoking has a detrimental effect on the periodontium, increasing the risk to develop deep pockets. The limitations of the study can include the relative reduced number of individuals in the study sample, the lack of information regarding the level of the plasma glucose. It can be possible that many of the diabetic patients to control well the level of the blood glucose, in this situation the periodontal condition being satisfactory as well. Secondly, the lack of information regarding the genetic influence on PD in our study group can also be a factor that affects the results. Other studies reported genetic susceptibility to PD [8] which was not taken in consideration. It can be concluded that tobacco use was an independent risk factor for periodontal disease in terms of deep pockets formation in this group of ageing Romanian subjects. The results support the implementation of population-based smoking cessation programs to reduce the prevalence of deep pockets in ageing populations.


