Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Toshiyuki Tamura1, Shigetoshi Yokoya2, Yohei Kamata2, Yukihiko Kinoshita3, Yasuhiko Tabata4 and Goichi Matsumoto*3
Received: April 23, 2018; Published: May 02, 2018
*Corresponding author: Goichi Matsumoto, Department of Oral and Maxillofacial Surgery, Kanazawa Medical University. 1-1 Daigaku, Uchinada, Kahoku-gunn, Ishikawa 920-0293, Japan
DOI: 10.26717/BJSTR.2018.04.001025
We report here the clinical effects of the sustained release of basic fibroblast growth factor (bFGF) from gelatin hydrogel in cases of periodontal disease with infrabony defects. Patients with moderate to advanced periodontitis were treated with a mucoperiosteal flap operation. At the time of surgery, each bone defect was filled with gelatin hydrogel incorporating bFGF (bFGF/gelatin hydrogel). Probing pocket depth (PPD), clinical attachment level (CAL), and radiographic bone level were evaluated before treatment and at 3, 6, and 12 months after treatment. There were significant improvements in clinical parameters after treatment. There were no adverse effects during the treatment period. Topical application of bFGF/gelatin hydrogel may promote periodontal regeneration in cases of chronic periodontitis with infrabony defects.
Keywords: bFGF; Gelatin hydrogel; DDS; Regeneration; Infrabony defects; Periodontitis; Clinical trial
Recent progress in periodontal biology has demonstrated that undifferentiated mesenchymal stem cells and progenitor cells exist within the periodontal ligament [1,2] Therefore, enhancing the biological potential of these cells facilitates periodontal tissue regeneration. Bioactive factors that stimulate cellular proliferation and differentiation, such as bone morphogenetic protein-2 (BMP- 2), platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), transforming growth factor beta (TGF-P), and basic fibroblast growth factor (bFGF), contribute to bone metabolism and to the organic inflammatory reaction, which is the healing process that takes place in wounds [3-5].
Many studies have shown that topically applying these bioactive factors to periodontal bone defects leads to regeneration of periodontal tissues [6-8]. We are particularly interested in bFGF, which has been shown to enhance the proliferation potency and chemotaxis of certain cells that originate in periodontal tissue and to enhance the healing of periodontal wounds [9-11]. Recent data gathered in a Phase II and III clinical trial regarding periodontal disease demonstrated that topical application of bFGF can successfully treat infrabony defects and class 2 furcation defects [12,13]. In this clinical experiment, hydroxypropyl cellulose was used as a vehicle to deliver bFGF in a controlled pattern of release. However, the half-life of the material proved to be short in the physiological environment. Therefore, a larger dosage is necessary for bFGF to be effective. In a search for materials that perform well, researchers have experimented with a variety of growth-factor delivery systems. They have incorporated growth factors into natural polymers, synthetic polymers, and ceramics, and they have experimented with physical entrapment, chemical immobilization, and other means to harness the growth factors into the delivery vehicles. Ikada et al. [14,15] have developed a drug delivery system (DDS) in which a growth factor that has been incorporated into gelatin hydrogel is released sustainably during the degradation of the gelatin hydrogel within the living body [16].
In their experiments with bone defects in animals, they found a significant increase in quantity of bone formed due to the bFGF-gelatin hydrogel [17,18]. In addition, Takayama et al. showed that topical application of bFGF/ gelatin hydrogel considerably enhanced periodontal regeneration in a primate model [19]. We have sought to extend our knowledge of effective treatments of bone loss and periodontal disease with the case report presented here. Our goal is to further develop clinically effective methods of topically applying bFGF/gelatin hydrogel to treat chronic periodontitis accompanied by infrabony defects.
For this clinical study, we recruited 23 patients (17 females and 6 males with a mean age of 50.3 years) who had moderate to advanced chronic periodontitis and who were scheduled to receive periodontal therapy at the Yokohama Clinic and Hospital of Kanagawa Dental University (Table 1). The study design and consent forms were approved by the ethics committee of Kanagawa Dental University. Criteria for enrollment in the study were:
a) a non-compromised systemic health condition and no contraindications for periodontal surgery;
b) a probing attachment loss equal to or greater than 4 mm; and
c) Clinical and radiographic evidence of the presence of an interproximal defect with an infrabony component. All study subjects had destruction of periodontal tissue and at least one 1-, 2- or 3-walled bone defect. This clinical trial was conducted according to the schedule shown in Figure 1. Before periodontal surgical treatment, each patient received an initial preparatory session, including oral hygiene instruction, scaling, and root planing.
Figure 1: Clinical trial schedule. The clinical trial was conducted in accordance with the clinical trial schedule.Periodontal examination consisted of measuring probing pocket depth and clinical attachment level.

The gelatin used in this study is an acid gelatin hydrogel that has an adjusted isoelectric point of 5.0 with moisture content of 95%. It is of cow bone origin (Nitta Gelatin Co., Japan). The acid gelatin was freeze-dried, and a safety identification test and a sterility test were conducted. The bFGF used Fiblast Spray (Kaken Pharmaceutical Co., Japan) which is a human recombinant protein and pharmaceutical authorized as a therapeutic agent for pressure sores and skin ulcers. The bFGF/gelatin hydrogel material was prepared before surgery. A bFGF water solution consisting of 250 Hg of bFGF dissolved in 300 μl of purified water was prepared. The bFGF-water solution was applied to a freeze-dried gelatin sheet and adjusted to contain 100 ng of bFGF for every 20 mg of gelatin and was left standing afterwards for 24 hours at 4 °C.
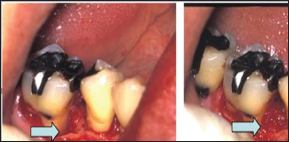
Following local anesthesia, intra-crevicular incisions were made extending to the neighboring teeth. Then, full-thickness mucoperiosteal flaps were raised vestibularly and orally. All granulation tissues were removed from defects and the roots were thoroughly scaled and planed by means of manual instruments. The defects and the adjacent mucoperiosteal flaps were then thoroughly rinsed with sterile saline, after which the defects werefilled with bFGF/gelatin hydrogel (Figure 2). After placing the hydrogel material, the flaps were repositioned and closed with sutures.
Figure 2: Application of bFGF/gelatin hydrogel to an infrabony defect. The mesial region of a mandibular rightfirst molar shows a 3-walled infrabony defect (arrow) (A). After granulation tissue was removed from the region, the defect was filled with bFGF/gelatin hydrogel (B).
All patients received antibiotics orally for 5 days following surgery. The sutures were removed 7 to 14 days after surgery. Recall appointments were scheduled weekly during the first 4 weeks after surgery and then once a month for the rest of the 1-year observation period. Safety evaluations were conducted, subjective symptoms were recorded, and objective findings were confirmed by interview and visual inspection.
The experimental outcomes of interest included soft-tissue and hard-tissue measurements of the intra-oral radiographs taken with a parallel technique:
a. Change in probing pocket depth (PPD)
b. Change in clinical attachment level (CAL)
c. Changes in the radiographic values of linear bone gain (LBG) and percentage of bone fill (BF)
The values of LBG and BF were determined by measuring intraoral radiographs in accordance with the methods of Nevins et al. [20]. LBG was determined by measuring the distance from the CEJ to the base of the defect at the baseline minus the distance from the CEJ to the base of the defect at each of the post-surgical time points. The radiographic rate of bone fill was calculated by dividing the LBG by the depth of the original bone defect.
For statistical evaluation of the change from baseline to 3, 6, and 12 months, we used the Wilcoxson signed-rank test.
PPD: probing pocket depth, CAL: clinical attachment level, LBG: radiographic linear bone gain, BF: radiographic bone fill
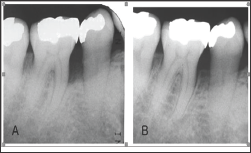
The characteristics of the subjects in this study are shown in Table 1. The bFGF dose ranged from 40 μg to 185 μg (mean 89.7 μg). All subjects completed the study; in all cases postoperative healing was uneventful. No allergic reactions, abscesses, infections, or other complications were observed during the study period. The pre-treatment and post-treatment clinical parameters are shown in Table 2. We compared the values of PPD, CAL, and LBG at 3, 6, and 12 months after treatment with the pre-treatment values. From before treatment to 12 months after treatment, the changes in mean PPD and mean CAL of treated sites were 3.8 ± 1.9 mm and 5.9 ± 2.0 mm, respectively. Also, at 12 months following treatment, hard tissue showed a mean LBG of 3.5 ± 2.1 mm and a mean BF of 61.8 ± 25.2%. The mean depth of bone defects was less at both 6 months and 12 months compared with the pre-treatment mean (Figure 3). These differences were statistically significant. A representative case treated with bFGF/gelatin hydrogel is shown in Figure 2. In this case, the 12-month radiograph showed good bone fill compared with the pre-treatment radiograph (Figure 4).
Figure 3: Changes of periodontal parameters. PPD: probing pocket depth, CAL: clinical attachment level, DB:depth of bone defect; *: significant difference from before treatment (p<0.05).
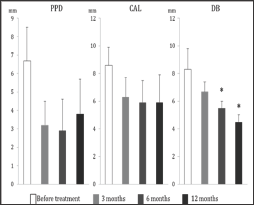
Figure 4: Dental radiographs. Preoperative radiograph of an infrabony defect on the mesial aspect of amandibular right first molar (A). At welve months after bFGF/gelatin hydrogel treatment (B).
Gelatin, a denatured collagen, is obtained by acid and alkaline processing of collagen isolated from bovine bone. This processing affects the electrical nature of collagen, yielding gelatin with different isoelectric points (IEPs) [15]. When mixed with positively or negatively charged gelatin, an oppositely charged protein ionically interacts to form a polyion complex. When bFGF that has been ionically complexed to an acidic gelatin hydrogel is placed in a target site, the bFGF will be released after a periodas a result of hydrogel degradation [21,22]. The degradation is controllable by changing the extent of cross-linking, which, in turn, affects the water content of the hydrogel. The rate of bFGF release accords well with the rate of hydrogel degradation.Gelatin hydrogel releases the protein drug without interfering with biological activity [16,22]. The optimum water content for promoting bone formation without disturbing it due to unabsorbed gelatin is 90-98% [23,24]. In the present study, the water content of the hydrogel was 95%. The bFGF release period associated with hydrogel degradation was expected to be about 4 weeks. We had no trouble placing bFGF/ gelatin hydrogel in the target site without it flowing out into the surrounding tissue.
Gelatin hydrogel has already been used to clinically deliver bFGF to treat ischemic limb disease and Bell's palsy [25,26]. The mechanisms by which bFGF facilitates periodontal regeneration are thought to include angiogenic activity and a mitogenic effect on mesenchymal stem cells within the periodontal ligament [3]. We hypothesize that periodontal regeneration is promoted by the sustained release of bFGF from gelatin hydrogel. In addition, we suspect that gelatin plays a role as a scaffold in the early stage of periodontal tissue regeneration.In our clinical study, a marked improvement of the infrabony defect was observed at 6 months after treatment. The radiographic and clinical parameters were stable at 12 months after bFGF/ gelatin hydrogel treatment. It has been reported that the clinical parameters and radiographic findings were stable at 6 years after 0.3% bFGF treatment. This may have a higher capacity for new alveolar bone regeneration than other periodontal regenerative therapies [27].
We evaluated the clinical effects of the sustained release of bFGF from gelatin hydrogel for the treatment of periodontal disease with infrabony defects. We treated 23 subjects with a mucoperiosteal flap operation. At the time of surgery, each bone defect was filled with bFGF/gelatin hydrogel, and this treatment led to improved clinical parameters, specifically, clinical attachment gain, probing depth reduction, and radiographic bone fill. We conclude by suggesting that the topical application of bFGF/gelatin hydrogel in the treatment of chronic periodontitis with infrabony defects or furcations is safe, leads to improvement of clinical parameters, and offers promise as a new avenue for periodontal regeneration. Since limited human clinical data are available, more studies with randomized control will be needed to fully evaluate the potential of bFGF/gelatin hydrogels for ehancing periodontal regeneration.


