Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Esin Akbay* and Mehmet Ali Onur
Received: May 29, 2018; Published: June 11, 2018
*Corresponding author: Esin Akbay, Department of Biology, Faculty of Science, University of Hacettepe, Ankara, Turkey
DOI: 10.26717/BJSTR.2018.05.001191
The purpose of this study is to develop the optimal method for heart decellularization using for tissue engineering. Several different chemical methods have been studied for decellularized tissues including heart. Nevertheless, there are not certain results about which combination of these methods may provide the best transplantable, effective scaffolds. In this study, a new modified decellularization method has been applied to heart including different concentrations of ionic and non-ionic detergents. To observe decellularization results histological analysis were performed. As a result, it was observed that chosen detergent solutions at low percentile concentrations and in short time duration showed efficient performance. Histological analysis indicated that samples were nuclei free in all groups. The results of Acridine orange (AO) and propidium iodide (PI) staining showed that there were a few viable attached cells.Hereby, results demonstrated that obtained tissue scaffolds have a well surface for cell attachment.
keywords: Decellularization, Heart, Scaffold, Tissue Engineering, Extracellular Matrix
Abbreviation: ECM: Extracellular Matrix, PBS: Phosphate Buffer Saline, AO: Acridine Orange, PI: Propidium Iodide
Today, although the incidence of heart failure continues to increase steadily, the number of donor organs needed for transplantation is insufficient. In this case, the regeneration of whole organs or using just as a scaffold mesh of the decellularized organs may theoretically overcome these problems. Extracellular matrix (ECM) provides a promising role for regenerative medicine and tissue engineering, because it is a natural scaffold material, it is released and produced by native cells of each tissues and organs [1]. Chemical, biological and physical methods have recently been utilized, separately and as a combination, to decellularized whole heart obtained from various rodent species [1,2]. These different methods are being concluded with a histologic view but that vary in ECM composition and other structural properties. However, it is not clear whether variation of ECM and other protein content and composition of decellularized heart meshes obtained from the different protocols will change the following cell seeding step or not. The most essential point is to organize the right methods for selecting the potential clinical utility of the decellularized heart scaffolds [3].
During the recent years, many decellularized tissues have been worked out for utilization as a pattern for tissue engineering approaches. Intact tissue decellularization methods are based on classic methods described before. However, instead of the basic agitation and diffusion, decellularization technique has been used singly to obtain scaffolds from thin and small tissues. Due to the layers and sophisticated intrinsic structures of tissues, decellularization needs more complicated processing. Here, the choice of decellularization reagents is critically essential since they can cause serious damage at the structure and composition of the obtained cell free scaffold. Selection of decellularization agents, together with the improvement of new protocols, have been considered to be most important steps of the protocol, because these agents can also damage to the natural ECM structure. All tissues have various intrinsic components. Thus, removing the cells from different tissues needs different decellularization agents procedure [2]. To achieve this, from various groups of decellularization agents, non-ionic and ionic detergents were chosen in this study. And by this paper a decellularization method for heart tissue has been shown to be able to preserve the scaffold being used as a pattern for subsequent cell seeding and detachments.
Male Wistar albino rats were used with the approval of the Animal Care and Use Committee, Hacettepe University, Ankara. After anesthesia, thoracotomy was performed and femoral vein was catheterized for phosphate buffer saline (PBS) perfusion. After that, all of the intra-heart blood was removed. At that time, heart was removed quickly and stored in PBS at 4°C till Decellularization protocol was applied. Methods that have been described previously were normally applied to decellularized various tissues. Steps in methods were modified for heart as shown in Table 1. After other analyses were applied, the most effective method was determined.
Table 1: Summary of modified decellularization protocols for heart tissue (h: hour) (In each step all samples were washed with PBS.)
Lyophilization procedure was applied to all decellularized heart samples with control groups. Lyophilized pieces of scaffolds from each tissue samples were stored in -80°C and used at a later time point for histological and immunological analysis.
Samples of decellularized scaffold and control heart tissues were randomly selected for H&E and Masson’s Trichrome staining. From these staining methods, H&E was used to indicate the existence of residual cells or cell fragments and Masson’s Trichrome was used to show collagen content of ECMs. Control and decellularized heart biopsies were fixed in Bouin solution. The paraffin sections were cut at 5 mm thickness and stained with H&E. All histological sections were viewed using an inverted microscope (Olympus IX70 Inverted Microscope, Japan).
All cell free decellularized scaffolds were placed in 24-well culture plates and sterilized under UV light for 45 min. After this step, scaffolds were soaked in 20μl culture medium (DMEM/F12 and 10% FBS, v/v) for 1 h. 250μlof L929 cell suspension with a density of 4×104 per cm2 cells/scaffolds were applied on all scaffolds. This step was repeated several times till cell free scaffolds covered totally with culture medium include cells (500μl).
AO/PI fluorescent staining was used for assessment of the viability of cells on scaffolds. Before staining, cell-scaffold constructs were transferred into 96-well. AO/PI solution (1/1, v/v) was prepared and it was used for staining. In the next step, the AO/ PI solution was applied to cell-scaffold constructs for 5 min. Green (AO) and orange (PI) fluorescence’s were both observed under fluorescence microscopy (Olympus IX70 Inverted Microscope, Japan)
In the first step of the decellularization process, the hearts lost some of their red-brown color and became flabbier, and the reagent (in the first concentration of SDS solution steps of all seven modified methods) became pinkish in appearance. As the reagent changes in each step, the hearts became whitish in appearance. (Figure 1A & 1B).
To make the scaffold implementable meshes for transplantation, lyophilization methods were applied to all groups of decellularized heart tissues (Figure 1C).
Histological analysis showed the removal of nuclei and nuclei residual in all groups. But except M3 in all other groups unsteady structure has been observed (Figure 2).
Figure 2: Microscopic images of rat native heart (a) and decellularized rat heart (b-h) with different concentration of detergents (0,1%, 0,5%, 1% of SDS and TritonX-100) for 1 h, 24 h, 48 h and 72 h. (H&E, × 200).

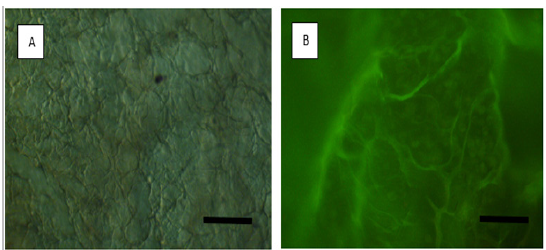
The living cells seeded on the scaffolds are shown in red and the dead cells are shown in green by AO/PI staining (Figure 3). The viability of the cells at different experimental groups was indicated healthier structure than that of the control groups of normal cell structures.
Figure 3: Attached and live cellmorphologies of cells on decellularized heart tissue scaffolds. (A) Control heart tissue and (B) representative images of AO/PI stained cells (green-sphere shape) on M3 decellularized heart tissue. Original magnification: X200.
In the last few years, cardiovascular tissue engineering has been showed as a new approach that incorporating two different disciplines; biomaterial chemistry and cell biology [3]. Unlike blood vessels or heart valves, heart muscle cell has no regeneration properties. Cardiac tissue engineering aims at yielding in vivo therapy for replacement contractile heart muscle constructs. Currently, a large number of cardiac tissue engineering methods are preferred embryonic chicken and neonatal rats for the isolation of heart muscle cells and for scaffolding materials [4,5]. Tissue scaffolds provide a temporal but crucial structural support for the cells due to their secretion and maintaining ability of their own ECM [6]. Also our observations indicated that in vivo heart mesh and optimal cell usage could be non-invasive and located therapy if provided with appropriate environmental cues.
In the few years, one of the most popular approaches is the engineering of in vitro myocardial tissue.Based on synthetic polymers or biological components, various heart muscle cells were obtained as 3-dimensional in vitro culture systems. Li et al. [7] demonstrated that heart muscle cells can attach to cell free scaffold meshes to form contractile cell-polymer structure. Bursac et al. [8] studied on cell-polymer bioreactor model system and applied electrophysiological analysis for comparing structural properties with natural cardiac tissue. Despite the potential clinical implementation, methods used to date have important drawbacks or limitations like deciding to the optimal size; succeed of plentiful cell attachment, cell viability and mechanical interaction. According to all mentioned issues above, we focused on the best way of decellularization methods that minimized the damage at ECM and also optimized the total mesh surface area availability by using lyophilization method for implantation and apply sufficient number of cells. M3 methods showed the best structure and cell attachment potential. The development of functional scaffold surface area might be a crucial step in accordance with a successful, cell-based restoration of damaged heart with a weak function.
This study was supported by Hacettepe University Foundation Project No: 0130161002.


