Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
William Davidson Urbain1, Philippe Jouvet1,2, Maria P Vélez3, Pierre Ayotte4 and Patricia Monnier*5
Received: June 20, 2018; Published: July 03, 2018
*Corresponding author: Patricia Monnier, MUHC Reproductive Center, 200-88 de Maisonneuve Blvd East, Montreal, Qc, H2L 4S8
DOI: 10.26717/BJSTR.2018.06.001322
Introduction:Medical devices used in Pediatric Intensive Care Units (PICU) are mostly made of plastic containing plasticizers that can leach out into the blood. We conducted a pilot study to assess exposure to phthalates in PICU.
Methods:Phthalates metabolites levels were measured in urine of critically ill children at admission and Day 4. Plastic medical equipment required for each patient was also recorded.
Results: All sixteen patients had high levels of phthalates metabolites in urine and infants had higher levels than adolescents. Despite plastic exposures, no increase in phthalates metabolites between admission and day 4 was observed.
Conclusion: Children in PICU have a high degree of phthalates exposure
Keywords: Children; Intensive Care; Phthalates; Prospective Study
Over the last 30 years, glass infusion containers were gradually replaced in intensive care units by plastic. However, unlike glass, these materials are not inert. More than 25% of all the plastics used in medical devices are made from polyvinyl chloride (PVC), a rigid product that is not easily malleable [1]. Plasticizers are added to PVC to make it flexible. Among the commercially available plasticizers, phthalates are the most commonly used. However, phthalates are not chemically bound to PVC and over time and use, they are released in the environment [2]. These synthetic chemical compounds have become ubiquitous in indoor and outdoor environments, in animals and in humans [3]. Of the 25 existing types of phthalates, di (2-ethylhexyl) phthalate (DEHP) is the only FDA-approved (US Food and Drug Administration) plasticizer in medical devices [4]. In intensive care units DEHP was detected in the following situations:
i) Medical Devices: intubation tubes and circuits fan, digestive tubes, plastic gloves, central catheters made of PVC, dialysis circuit tubing, tubing of circulatory support (extracorporeal circulation (ECC) and extracorporeal membrane oxygenation (ECMO));
ii) Solute infusion liquids due to interaction between the plastic bags, tubing and some commonly used products such as: parenteral nutrition (especially intralipid), midazolam, fentanyl, blood products (red cell concentrate, platelets, fresh frozen plasma) [5,6].
While the average daily exposure to DEHP is estimated at 0.27 mg per day [1], the continuous exposure in intensive care units can result in an intake of 10 to 20 mg per day of DEHP [5]. The FDA has set a tolerable intake level of DEHP for parenteral exposure of 0.6 mg/kg/day [4]. However, the amount of phthalates released by medical devices does not appear to vary according to age groups, so that the dose of DEHP reported to the patient’s weight is greater as a child is younger, which explains that children are more vulnerable compared to adults [7]. Thus, the most exposed children to high doses of DEHP are those on life support, transfusion and under extracorporeal circulation [1]. Furthermore, an increase in body temperature appears to increase the amount of phthalates released from plastics [8].
Exposure to phthalates can affect different organs, such as brain, liver, lungs, heart and endocrine system, and have short and long term consequences [1,9]. Although no prospective studies have reported a causal relationship between exposure to medical devices containing phthalates and diseases in children, several studies have investigated the association between phthalates and certain medical conditions. Among these studies, a large study including 449 children treated in pediatric intensive care units (PICU) have shown an association between attention deficit observed in children 4 years after critical illness and iatrogenic exposure to DEHP metabolites during intensive care. We conducted a pilot study, during the same period as S Verstraeted et al. [9], aimed to quantify the level of exposure to phthalates of children treated in a single Canadian PICU.
This prospective study was conducted in the PICU of Sainte- Justine Hospital, a tertiary medical surgical children’s hospital, between October 2009 and July 2012. Enrolled patients had to be hospitalized at the intensive care unit besides having an endotracheal tube for a period of at least three days. Patients who had cardiac surgery with extracorporeal circulation and those undergoing extracorporeal membrane oxygenation (ECMO) were excluded due to the already proven high exposure [10], chronic home care entailing the use of a medical device likely to contain plasticizers (catheters, urinary catheters, tracheostomy, ...) or prior hospitalization within one month before the current admission in another department, excluding an elective surgery, were also part of exclusion criteria. A a posteriori exclusion criteria was a creatinine blood level above normal range as a renal failure results in misinterpretation of plastics metabolites excretion. Informed consent was obtained from each patient or patient’s family and the study was approved by the Research Ethics Board of the Sainte- Justine University Hospital.
Exposure to phthalates was evaluated at two times: once on arrival in the PICU (during the first twelve hours following admission) and once three days later. The exposure variable was analyzed according to the results of urinary metabolites and the patient’s age group, while considering the medical devices to which children were exposed.
The dependant variable is the difference of urinary metabolite levels between the third day following admission and the first day. The choice of an observation period of three days is based on the study of J Weuve et al. [11] showing stability of repetitive assays between 6 and 72 hours starting from the third day in neonatal intensive care units.
Patients were categorized into three age groups: Group 1: infants (up to 2 years), Group 2: children (from 2 to 12 years), Group 3: teenagers (from 12 to 18 years). Ethnicity, gender and weight at admission were recorded and also considered as covariates of interest. Apart from the above variables, other data related to the existence of a chronic condition, the admitting diagnosis, the PIM 2 score (predictive score of mortality in pediatric intensive care) [12], organ failure score for the first three days (PELOD score) [13] and changes in renal and hepatic function according to the medical record.
The urine collection is part of the standard care in intensive care. It was provided by urinary pockets or a urinary catheter, according to medical decision. Sample collection devices were prescreened for DEHP metabolites. Urine samples (2ml) were placed in a polypropylene container and frozen at minus 20 °C. The containers used for sampling had been previously checked to be free of contamination by phthalates metabolites. Chemical analyses were carried out by the Centre de toxicologie du Québec, located in the Institut national de santé publique du Québec, which is accredited by the Standards Council of Canada under ISO 17025 and CAN-P-43.Mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl- 5-oxoyhexyl phthalate (MEOHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), and mono-2-methylcarboxyhexyl phthalate (MMCHP), metabolites of DEHP were analyzed.
The analytical method for the determination of phthalates monoesters in urine was previously described [14]. Briefly, urine samples were enriched with labeled internal standards (MMP- 13C4, MEP-13C4, MCHP-13C4, MBzP-13C4), MEHP-13C4, MEHHP- 13C4, MEOHP-13C4, MECPP-13C4, MOP-13C4, MCPP-13C4 and MNP-13C4). The urinary metabolites were then hydrolyzed with β-glucuronidase enzyme. Thereafter, the samples were acidified with H3PO4 50% and were extracted. The extracts were evaporated to dryness and dissolved in demineralized water. The samples were then analyzed by liquid chromatography with a tandem mass spectrometer (MS/MS) in the MRM mode with an electrospray ion source in the negative mode. To reflect the degree of urine dilution, the measured concentrations were also corrected by urinary creatinine level. As creatinine is produced and excreted at a constant rate over 24 hours, urinary creatinine corrections are helpful to offset the effect of urinary dilution, as well as the variations caused by lean body mass [15,16]. Concentrations were reported in units of micrograms per liter (μg/L) and the limits of detection (LOD) reported were 0.04μg/L for MECPP, 0.06μg/L for MEHP, 0.07μg/L for MCPP, 0.08μg/L for MBzP and MEOHP, 0.1μg/L for MiBP, MnBP, MCHP and MNP, 0.2μg/L for MOP, 0.3μg/L for MEP and MEHHP and 4μg/L for MMP.
The Wilcoxon signed-rank test was used to compare the levels of phthalate metabolites between day 1 (arrival day) and day 4(95% confidence interval). Spearman correlation test was used to evaluate the relation between urinary levels of these chemicals and the weight of the patient (95% confidence interval). Significant statistical level: p 0.05.
Between October 2009 and July 2012, 2642 patients admitted to PICU were screened. Fifty two patients fulfilled the inclusion criteria (Figure 1) and had urine sample at day 1 collected. However, in 32 cases, it was not possible to collect urine sample at day 4 for various reasons (extubation, departure of the PICU or early death). Therefore, a total of 20 patients had the two required urine samples collected (Day 1, and Day 4). Of these 20 eligible patients, four were further excluded due to creatinine blood level higher than normal, suggesting renal failure. The remaining 16 patients were distributed as follows: 8 infants aged between 0 and 2 years, 6 children aged between 2 to 12 years and 2 adolescents of respectively 12 and 15 years old. The clinical characteristics of the patients and the medical procedures performed between day 1 and day 4 are described in Tables 1 & 2.
a Data are expressed as mean ± standard deviation for continuous variables and as n (%) for categorical ones.
bLenght of stay in PICU.
PELOD: Pediatric Logistic Organ Dysfunction score (13), MV: mechanical ventilation, PIM2: Pediatric Index of Mortality (12).
a Expressed as mean ± standard deviation.
PVC: Peripheral venous catheter, CVC: Central venous catheter includes umbilical arterial catheter, EVD: External ventricular drain.
Table 3: Concentration of urinary creatinine, phthalate metabolites, by age group and day of collect (mean ± STD). Patients were categorized into three age groups: Group 1: infants (up to 2 years). Group 2: children (from 2 to 12 years). Group 3: teenagers (from 12 to 18 years).
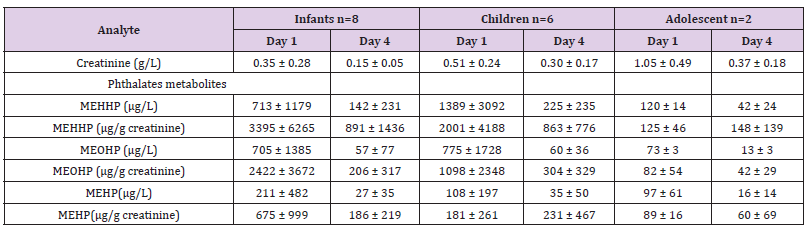
Six DEHP metabolites were detectable in more than 98% of the samples (MnBP, MEP, MBzP, MEHP, MEOHP, MEHHP), while MCPP was detectable in 82%. Concentrations of MCHP (detected in 12 samples), MMP (detected in 3 samples) and MOP (detected in 2 samples) were generally lower than the LOD, while the concentrations of MNP were under the LOD in all samples. Since MEHHP, MEOHP and MEHP, DEHP metabolites, were detected in all samples and because they are the most frequently studied in the literature [11,17,18] only these three metabolites were considered for the remainder of the study. Phthalate metabolite concentrations varied widely among patients and generally did not increase between the arrival day and day 4. Geometric mean concentrations were even lower on day 4 than they were on admission day without statistical significance (Table 3). DEHP metabolites urine levels were lower in older children (Table 3). The association between urinary concentration on day 1 and weight at admission was significant for MEHHP, MEOHP (p= 0.048 and p=0.010, respectively) but not for MEHP (p=0.915). Spearman tests showed no correlation between each concentrations of DEHP metabolites and PIM 2 and PELOD scores. However, there was a trend between PELOD scores and MEHP urinary concentrations on day 1 (r = 0.435, p = 0.093).
We observed high level of DEHP metabolite in urine of critically ill children and non-significant lower levels of plastics metabolite in urine at day 4 when compared to admission levels. We also report a highly significant inverse correlation between the weight of observed patients and urinary plastics metabolite concentrations. Infants had concentrations of MEHHP and MEOHP approximately 1.4 times higher than children and about 17 times higher than adolescents (Table 3). MEHP concentrations in infants were 2 and 5 times higher when compared to children and adolescents respectively. The level of DEHP metabolites in urine of critically ill children were much higher than levels observed in healthy children in Quebec [19]. The average urinary MEHP concentrations on day 4 of our patients aged from 0 to 24 months (141.5μg/L) were above 30 times higher than those observed in healthy children in US (4.6μg/L) [20].
Several factors could explain the correlation between the age of patients and urinary excretion of DEHP metabolites. First, the younger the child, the higher the surface/volume ratio is. Therefore, even if the exposure in PICU remains the same regardless of weight or age, it is still proportionally higher in patients whose weight is lower. Infants also have a more important oral exposure due to their higher caloric intake per kg of body weight when compared to older children or adolescents [7]. Furthermore, frequent oral contacts such as mouthing and sucking objects containing or contaminated with phthalates or BPA (toys, pacifiers, plastic containers, etc.) in addition to ingestion of contaminated dust, breast milk, infants formula, cow’s milk or food packaging can also lead to increased exposure in this population [21]. However, these behavioral patterns and sources of exposure can only have influenced the concentrations on day 1, since they are commonly inherent to the normal environment of the child and almost absent in the PICU. The correlation observed in this study between age and urinary DEHP metabolites reinforces the idea that infants and young children represent a population at considerably higher risk. Thereby, concerns about the effects of phthalates on human health are even greater for newborns, especially because of their different physiology and their immature metabolic pathways that could lead them to longer and stronger exposure to phthalate.
In this study, we found no significant correlations between urinary levels of DEHP metabolites in patients admitted to the PICU, when comparing levels on the day of arrival and on the third day following admission. However, the levels found on day 4 usually appeared lower than those measured at the day of admission. This was also observed by S Verstraeted et al. [9] and can be explained by higher plastic released from medical devices used in another department, on ambulance transport or in the emergency room prior to PICU admission than plastic release during PICU stay. As the length of stay in PICU is often of a short duration (average of 206 hours for patients in this study), children are only exposed to high doses of DEHP for a few days. The health impact of this short but high exposure seems important. B Schneider et al. [22] have first demonstrated a relation between DEHP and the increase of conjugated bilirubin in 29 children on ECMO. S Verstraeted et al. [9] assessed neurocognitive development on 224 infants and concluded that iatrogenic exposure to DEHP metabolites during intensive care was independently associated with the important attention deficit observed in children 4 years after critical illness. A Stroustup et al. [23] recently observed that exposure to phthalates mixtures was associated with improved attention and social response in premature neonates, suggesting that the impact of phthalate exposure on neurodevelopment may follow a non-linear trajectory, perhaps accelerating the development of certain neural networks. The strength of our study is the prospective data collection and the rigorous DEHP metabolites analysis. The weaknesses include (i) the small number of patients included and the single center design that limit generalizability but this study was designed to detect the level of exposure to plastics in critically ill children with usual PICU management.
This pilot study showed that children hospitalized in PICU have a degree of exposure to DEHP considerably higher than those in the general population. This study also confirms that young children represent a population at risk of exposure to DEHP, while their urinary concentrations are significantly higher than those of older children and adolescents. Considering that DEHP could have an impact on the development of infants and particularly for neuro developmental health, extra care should be taken by caregivers to reduce children exposure to plasticizers in PICU. Therefore, future studies are needed to demonstrate the actual effects on health of phthalates. It would also be interesting to determine whether a short but high exposure to these chemicals can cause permanent damages to the health and human development.


