Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Princy Agarwal*1, Rajat Vaishnav1 and Mahendra Singh Ranawat2
Received: June 25, 2018; Published: July 09, 2018
*Corresponding author: Princy Agarwal, Department of Quality Assurance, Bhupal Nobles Institute of Pharmaceutical Sciences, BNUniversity,Sevashram Road, Udaipur-313001, Rajasthan, India
DOI: 10.26717/BJSTR.2018.06.001372
YogarajGugguluvati is one of the most commonly used polyherbal formulation in Ayurvedic clinical practice since ages for the ailments of various bone disorders.
Objective: The present work was carried out in order to evaluate comparatively three different and popular formulations of YogarajGugguluVati to confirm their quality and also to highlight the variations that may be present in them.
Methods: Comparative assessment of three different and popular marketed formulations of YogarajGugguluVati was carried out for their organoleptic, pharmaceutical, physicochemical and phytochemical properties as per the methods prescribed in Pharmacopoeias.
Results:The observations reveal that all the parameters of three brands of YogarajGugguluVati had considerable differences in their values. The value of water soluble and alcohol soluble extractives of Brand A was very much higher and lesser than the standard values respectively. The pH of all the tablets was also higher than that of the standard values. The Hardness and Disintegration time of the tablets of Brand B was also very high than the other two brands. The tablets of Brand A also failed the weight variation test.
Conclusion: It can be concluded from the overall results that phytochemical and analytical evaluation and standardization of all the formulations should be performed strictly on all formulations so as to optimize the final product according to the standards, which would otherwise affect the therapeutic activity of the product.
Keywords: Quality Evaluation; YogarajGugguluVati; Pharmaceutical Evaluation; Physico-Chemical Evaluation; Phytochemical Evaluation; Heavy Metal Analysis
Abbrevations: API: Ayurvedic Pharmacopoeia of India; IP: Indian Pharmacopoeia; Pb: Lead; Cd: Cadmium
Guggulu is a herb popular in Ayurveda which is used to treat various ailments. It is an oleogum resin that spontaneously exudes due to the injury of the bark of Commiphora wightii. Guggul, the Commiphora mukul, is a small thorny tree native to the Middle East and the Indian plains. The name Guggul means “one that protects from disease”. Guggul is one of the “broad spectrum” health products with a wide range of benefits. There are five different varieties of Guggulu described in Ayurvedic texts. However, two of the varieties, namely, Mahisaksa and Kanaka Guggulu are generally preferred for medicinal preparations. Mahisaksa Guggu lu is dark greenish brown and Kanaka Guggulu is yellowish brown [1-3]. Yogaraj guggulu is a polyherbal formulation consisting of 29 ingredients with Guggulu as the major one. It acts by vitiating the Tridoshas. It is used traditionally for detoxifying, treating obesity, joint pain, arthritic conditions, muscle aches, rheumatism and gout. It increases digestion power, complexion, strength and immunity [2,4].
Quality control of the herbal formulation and drug is important to justify its acceptability in the modern drug system. The change from batch to batch begins with the collection of the raw materials themselves in the absence of any reference standard for identification. WHO has emphasized the need to ensure quality control of medicinal plant products using modern techniques and applying appropriate standards and parameters. The present work was carried out to standardize and evaluate the pharmaceutical, physicochemical and pharmacognostical properties of three different marketed brands of Yogaraj gugguluvati.
The following marketed Yogaraj gugguluvati preparations were used in the present study. Brand A (Batch No. SB 0528), Brand B (Batch No. E-1601), Brand C (Batch No. #A-YRG008). All brands of the Yogaraj gugguluvati were procured from the local market from the registered Ayurvedic Pharmacy.
All the organoleptic properties viz. color and odor of the drug were performed as per standard procedure and noted down.
Pharmaceutical parameters like Weight variation, Thickness, Diameter, Hardness, Friability and Disintegration time were determined as per standard protocols.
Determination of Weight Variation: 20 tablets were weighed at random and the average weight of the tablets was calculated. Then the individual weight of the tablet was compared with the average weight.
Acceptance criteria: Not more than 2 individual tablet weights deviate from the average weight by more than the deviation stated in the Pharmacopoeia (Table 1).
Determination of Thickness And Diameter: The crown tablet thickness and diameter of an individual tablet was measured with a Digital Vernier caliper, which permits accurate measurements and provides information on the variation between the tablets.
Determination of Hardness: Hardness of a tablet can be measured by using Monsanto or Pfizer tablet hardness testers. We have made the use of Monsanto hardness tester.
Determination of Disintegration Time: For the disintegration test one tablet was placed in each tube, the guided disc was placed above each tablet and the basket rack was positioned in 1 L beaker containing water at 37±2⁰C. For compliance with USP standards the tablets must disintegrate and all particles must pass through the 10 mesh screen in the specified time. If any residue remains it must have a soft mass with no palpably firm core. In the case of Ayurvedic tablets the disintegration time required for five tablets should not be more than 15 minutes, unless otherwise stated in the monograph.
Physicochemical parameters like moisture content (Loss on Drying), pH, total ash, acid Insoluble ash, water‑soluble extractive, alcohol soluble extractive values of all three samples were determined as per standard protocols. All the procedures are described as folloWS:
Determination oF Moisture Content/ Loss on Drying (LOD): An accurately weighed 5g of polyherbal formulation powder was taken in a tarred evaporating dish. The crude drug was then heated at 105⁰C in an oven for 3 hours. The drying and weighing was continued at half an hour interval until difference between two successive weighing corresponded to, not more than 0.25 per cent. Percentage moisture content of the sample was calculated with reference to the air dried powdered drug material.
Formula for calculation:
% LOD=(W2-W3)/(W3-W1 )×100 %
Where,
W1 = weight of container (g)
W2 = weight of container + wet sample (g)
W3= weight of container + dried sample (g)
W2 – W3= weight of moisture
W3- W1= weight of dried sample
Determination of Loss on Ignition (LOI): An accurately weighed 5g of polyherbal formulation powder was taken in a previously ignited and tared silica crucible and was heated in the oven at 105⁰C overnight (or the previously dried sample can also be used). The crucible was cooled and reweighed. The crucible was then placed into the furnace tray and was ignited in the Muffle Furnace at 500⁰C for about 4 hrs. The sample was then cooled in a dessicator for 30 min. and reweighed with the ash in it (WA). The observations were noted.
Formula for calculation:
% LOI=(WS-WA)/(WS-WC )×100 %
Where,
WC = weight of crucible (g)
WS = weight of sample (g)
WA = weight of ash (g)
Determination of Total Ash: An accurately weighed 3 g of the sample was taken in a previously ignited and tared silica dish/ crucible. The material was evenly spread and ignited in a Muffle Furnace by gradually increasing the temperature to not more than 450⁰C - 600⁰C till the carbon free ash was not obtained. The Total Ash value was calculated with reference to the air-dried powdered drug material.
Formula for calculation:
%Total Ash = Weight of Ash/Weight of sample taken×100%
“Determination of Acid Insoluble Ash: Ash above obtained, was boiled for 5 min with 25ml of 1M Hydrochloric acid and filtered using an ash less filter paper. Insoluble matter retained on filter paper was washed with hot water and filter paper was burnt to a constant weight in a muffle furnace. The percentage of acid insoluble ash was calculated with reference to the air-dried powdered drug material.
Formula for calculation:
% Acid-Insoluble Ash = Weight of acid in soluble residue/ Weight of sample taken×100%
Determination of Water Soluble Ash: 1g of ash obtained in total ash experiment was boiled for 5 min with 25ml water and insoluble matter collected on an ashless filter paper which was then washed with hot water and ignited for 15 min at a temperature not exceeding 450⁰C in a muffle furnace. Difference in weight of ash and weight of insoluble matter was determined as difference represents the value. The percentage of water insoluble ash was calculated with reference to the air-dried powdered drug material.
Formula for calculation:
% Water Soluble Ash = Weight of water soluble residue/ Weight of sample taken×100%
Determination of Extractive Values
(a) Determination of Alcohol Soluble Extractives: 5 gm of churna was accurately weighed and placed inside a glass stoppered conical flask. It was then macerated with 100ml of ethanol. The flask was shaken frequently during the first 6 hours and was kept aside without disturbing for 18 hours. It was then filtered and about 25ml of filtrate was transferred into a tared flat-bottomed shallow dish and was evaporated to dryness on a water bath. It was then dried to 105°C for 6 hours, cooled and finally weighed. The percentage of Alcohol Soluble extractives was calculated with reference to the air-dried powdered drug material.
Formula for calculation:
% Alcohol Soluble Extractive = (Weight of residue×100×100)/ (25×Weight of sample taken) %
(b) Determination of Water Soluble Extractives: Proceed as directed for determination of Alcohol–Soluble Extractive, using chloroform water (2.5 ml chloroform in purified water to produce 1000 ml) instead of ethanol.
(c) Determination of pH Value: The powder sample of Yogaraj gugguluvati was weighed to about 5g and immersed in 100 ml of water in a beaker. The beaker was closed with aluminum foil and left behind for 24 hour s in room temperature. Later the supernatant solution was decanted into another beaker and the pH of the formulation was determined using a calibrated digital pH meter.
(d) Phytochemical Evaluation
The aqueous and alcoholic extracts of the respective formulations were prepared and were subjected to preliminary phytochemical screening. These tests reveal the presence of various bioactive secondary metabolites which might be responsible for their medicinal attributes. Methods for preliminary qualitative phytochemical tests of the plant extracts are given below in the Table 2.
Determination of Heavy Metals (Lead and Cadmium)
Method (Direct Calibration Method): Three reference solutions of the element being examined having different concentrations were prepared covering the range recommended by the instrument manufacturer. Separately the corresponding reagents were added for the test solution and the blank solution was prepared with the corresponding reagents. The absorbance of the blank solution and each reference solution were measured separately, and the readings were recorded. A calibration curve was prepared with the average value of 3 readings of each concentration on the ordinate and the corresponding concentration on the abscissa. A test solution of the substance being examined was prepared as specified in the monograph. The concentration was adjusted such that it falls within the concentration range of the reference solution. The absorbance was measured 3 times, and the readings were recorded and the average value was calculated. The mean value was interpolated on the calibration curve to determine the concentration of the element.
Preparation of Lead Standard Solution: Lead standard solutions were prepared from Stock solution (1000 ppm Sisco Research Laboratories Pvt. Ltd. stock solution). Standard solutions of concentrations, 2, 4, 6, 8 and 10 ppm were prepared. The absorption of standard solution measured at 217 nm using hallow cathode lamp as a light source & air acetylene blue flame on Atomic absorption Spectrophometer.
Preparation of Cadmium Standard Solution: Cadmium standard solutions were prepared from Stock solution (1000 ppm Sisco Research Laboratories Pvt. Ltd. stock solution). Standard solutions of concentrations 0.2, 0.4, 0.6, 0.8 and 1.0 ppm was prepared. The absorption of standard solution measured at 228.8 nm using hallow cathode lamp as a light source & air acetylene blue flame on Atomic absorption Spectrophometer.
Preparation of Test Solution: Weigh accurately about 0.5 g of the coarse powder of the substance being examined, transfer into a casparian flask, add 5-10 ml of the mixture of nitric acid (HNO3) and perchloric acid (HCIO4) (4:1), add a small hopper on the flasktop, macerate overnight, heat to slake on the electric hot plate, keep somewhat-boiling, if brownish-black in color, add again a quantity of the above mixture, continuously heat till the solution becomes clear and transparent, then raise temperature, heat continuously to thick smoke, till white smoke disperse, the slaked solution becomes colorless and transparent or a little yellow, cool, transfer it into a 50 ml volumetric flask, wash the container with 2% nitric acid solution (HNO3), add the washing solution into the same volumetric flask and dilute with the same solvent to the volume, shake well. Prepare synchronously the reagent blank solution according to the above procedure.
Determination: Measure accurately 1 ml of the test solution and its corresponding reagent blank solution respectively, add 1 ml of solution containing 1% NH4H2PO4 and 0.2% Mg(NO3)2, shake well, pipette accurately 10-20 μl to determine the absorbance.
Sample Analysis: The analysis of the digested samples were carried out using an Atomic Absorption Spectrophotometer (EC Electronics Corporation of India limited AAS Element AS AAS4141) for Lead and Cadmium. The instrumental conditions for Lead analysis are depicted in Table 3.
Organoleptic Evaluation
The observations for the organoleptic evaluation of three brands of Yogaraj gugguluvati are reported in Table 4.
Pharmaceutical Evaluation
The observations for the pharmaceutical evaluation of three brands of Yogaraj gugguluvati are reported in Table 5.
Physico-Chemical Evaluation
The observations for the physico-chemical evaluation of three brands of Yogaraj gugguluvati are reported in Table 6
Phytochemical Evaluation
The observations for the phytochemical evaluation of three brands of Yogaraj gugguluvati are reported in Table 7.
Determination of Heavy Metals (Lead and Cadmium)
The observations for the heavy metal determination of three brands of Yogaraj gugguluvati are reported in Table 8 and Graph 1.
Graph 1: Graphs for various Pharmaceutical and Physico-chemical parameters of different brands of Yogaraj gugguluvati.
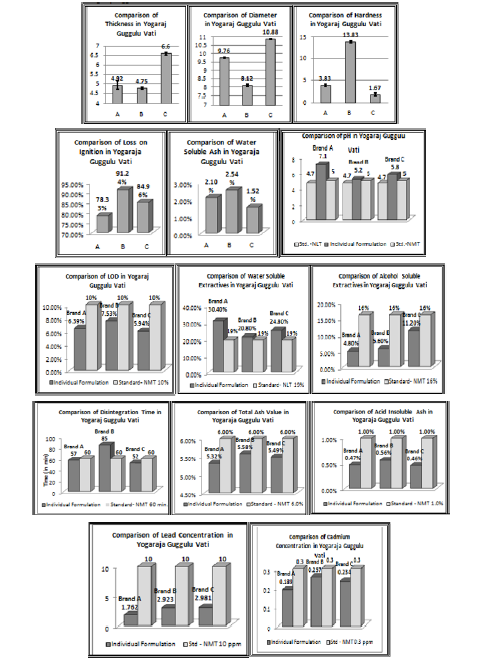
Yogaraj gugguluvati of Brand A was of solid state of Greyish brown color. This preparation had pH value of 7.1, and Loss on drying value of 6.39% w/w. Preparation had Alcohol soluble extractives and Water soluble extractives values of 4.8% w/w and 30.4% w/w respectively. The tablets failed the Weight variation test as per IP standards. The mean value and standard deviation of Thickness and Diameter of the tablets were 4.92 ± 0.23 and 9.76 ± 0.02 respectively. The tablet had Hardness value of 3.83 ± 0.289. The Disintegration time of the tablets was 57 min. respectively. It had Total Ash value of 5.32% w/w, and Acid insoluble ash and Water soluble ash value of 0.47% w/w and 2.1% w/w respectively. Loss on ignition was found 78.33% w/w. The concentrations for heavy metals Lead and Cadmium were found to be 1.762 and 0.189 respectively which were within the prescribed limits. Phytochemical screening revealed the presence of Carbohydrates, Steroids, Tannins and Terpenoids in both the extracts and of Alkaloids in alcoholic extract only.
This was in concordance with study by Ashima N Amin where chronic pyelonephritis with hydronephrosis was the most common lesion followed by multicystic renal dysplasia[5]. Chronic pyelonephritis was also the most common lesion in other studies like Kotta Devender Reddy et al.Aiman et al., Dr Bharti Devi et al., Shanmugaswamy et al.& Dr Ajay Kumar[1-6]This is in variation with findings of study done by Muhammad Raffique where the most common indication for nephrectomy cases in non-neoplastic lesion was renal stone disease[8].In our study we had 10 cases (14.08%) of Xanthogranulomatous pyelonephritis. Our findings were comparable with studies done by Aiman et al. (8 cases, 5.7%), Kotta Devender Reddy et al., (8 cases,10.4%). In our study out of 10 cases of Xanthogranulomatouspyelonephritis,6 patients were females showing female preponderance[1,7].We had 3 cases (4.22%) of Tuberculous pyelonephritis. This was comparable with Kotta Devender Reddy et al. 6 cases i.e. 7.8%, Ashima N Amin - 3 cases i.e.4.3%, Dr Bharti Devi thaker 1 case i.e. 1.4 [1,5,6]However in studies done by Swarnalath Ajmera and Shanmugaswamy et al there were no cases of tuberculous pyelonephritis [3,4].
Yogaraj gugguluvati of Brand B was of solid state of Blackish brown color. This preparation had pH value of 5.2, and Loss on drying value of 7.53% w/w. Preparation had Alcohol soluble extractives and Water soluble extractives values of 5.6% w/w and 20.8% w/w respectively. The tablets passed the Weight variation test as per IP standards. The mean value and standard deviation of Thickness and Diameter of the tablets were 4.75 ± 0.057 and 8.12 ± 0.074 respectively. The tablet had Hardness value of 13.83 ± 0.289. The Disintegration time of the tablets was 85 min. respectively. It had Total Ash value of 5.58% w/w, and Acid insoluble ash and Water soluble ash value of 0.56% w/w and 2.54% w/w respectively. Loss on ignition was found 91.24% w/w. The concentrations for heavy metals Lead and Cadmium were found to be 2.923 and 0.257 respectively which were within the prescribed limits. Phytochemical screening revealed the presence of Carbohydrates, Steroids, Tannins and Terpenoids in both the extracts; Phenols in aqueous extract only and of Alkaloids in alcoholic extract only.
Yogaraj gugguluvati of Brand C was of solid state of Dark brown color. This preparation had pH value of 5.8, and Loss on drying value of 5.94% w/w. Preparation had Alcohol soluble extractives and Water soluble extractives values of 11.2% w/w and 24.8% w/w respectively. The tablets passed the Weight variation test as per IP standards. The mean value and standard deviation of Thickness and Diameter of the tablets were 6.60 ± 0.082 and 10.88 ± 0.02 respectively. The tablet had Hardness value of 1.67 ± 0.289. The Disintegration time of the tablets was 52 min. respectively. It had Total Ash value of 5.49% w/w, and Acid insoluble ash and Water soluble ash value of 0.46% w/w and 1.52% w/w respectively. Loss on ignition was found 84.96% w/w. The concentrations for heavy metals Lead and Cadmium were found to be 2.981 and 0.234 respectively which were within the prescribed limits. Phytochemical screening revealed the presence of Carbohydrates, Steroids, Tannins and Terpenoids in both the extracts and of Alkaloids in alcoholic extract only.
Thus, all the parameters of three brands of Yogaraj guggulu Vati had considerable difference in their values. The value of water soluble and alcohol soluble extractives of Brand A i.e. 30.4% and 4.8% was very much higher and lesser than the standard values of 19% and 16% respectively. The pH of all the tablets was also higher than that of the standard values. The Hardness and Disintegration time of the tablets of Brand B was also very higher than the other two with the value of 13.83 ± 0.289 and 85 min. respectively. The tablets of Brand A also failed the weight variation t
The results of the present study clearly indicate that there is no uniformity in preparation of formulations. Organoleptic evaluation shows less similarity between formulations; Physicochemical analysis data depicts there are considerable differences in the quality parameters of all formulations and some parameters even don’t comply with the standard values given in different Pharmacopoeias. Hence, all these factors lead to variation in efficacy of drug formulations.
Therefore, we conclude that there is an urgent need to make more stringent quality control parameters and standardize the procedures for drug formulations from different reference books in order to reduce variation among all the Ayurvedic preparations.
Author is thankful to his guide and co-guides for their encouragement towards research work. Also giving thanks and appreciates to Dr. H.S. Purohit, Professor and HOD and Dr. Gajanand Jat, Assistant professor, Department of Agricultural Chemistry and Soil Sciences, Rajasthan College of Agriculture, Udaipur, Rajasthan for their timely suggestions and for providing necessary facilities to carry out the research work. Author is also thankful to Dr. Ram Pal Somani, Former Assistant Drugs Controller (Ayurveda), Govt. of Rajasthan, for suggesting to work in area of Quality assurance of Herbal drug.


