Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Lorenzo Pignataro1, Tullio Ibba1, Paola Marchisio2 and Sara Torretta1*
Received: July 11, 2018; Published: July 18, 2018
*Corresponding author: Sara Torretta, Department of Clinical Sciences and Community Health, University of Milan, Otolaryngology Unit, Italy
DOI: 10.26717/BJSTR.2018.07.001440
This paper aims at defining the therapeutic role of (adeno)tonsillectomy in children affected by recurrent acute tonsillitis (RAT), and at drawing some practical implications based on the current evidence. A literature search was performed to find pertinent study accessible by means of a MEDLINE search (accessed via PubMed). 15 papers were selected for literature analysis. The evidence suggests that although significant, the effect of tonsillectomy in children with moderate to severe RAT is modest and limited to 12 months post-operatively. In the case of patients with mild symptoms, it seems that the benefits are not sufficient to balance the disadvantages of the procedure. This can be explained by the fact that the procedure is intrinsically characterised by pharyngeal pain and that the surgical removal of a palatine tonsil does not exclude the subsequent onset of successive episodes of pharyngitis. An analysis of the literature indicates that the efficacy of (adeno)tonsillectomy in treating pediatric RAT is generally limited and relatively transient.
Keywords: Tonsillectomy, Children, Tonsillitis, Pharynx, Tonsils
Acute pediatric pharyngotonsillary inflammation is very frequently encountered in clinical practice, and its symptoms often limit children’s everyday activities. Furthermore, a number of children have recurrent or persistent symptoms characterised by a succession of acute infections associated with fever, pharyngeal pain, general malaise and, respiratory sleep disorders, and the limitations on their everyday activities cause them to miss school and prevent their parents from going to work. In such cases, (adeno)tonsillectomy can be considered, although it is necessary to consider the possible risks of surgery, including those related to the use of general anesthesia [1-3].
Although probably not the only cause, the palatine tonsils certainly play a major role in the genesis of episodes of recurrent acute pharyngeal inflammation, which means that, even if it cannot eliminate the risk, tonsillectomy could theoretically prevent further episodes of pharyngeal pain and/or reduce the severity of subsequent infections, thus improving the patient’s quality of life [4]. However, it is still not clear whether an accompanying adenoidectomy can lead to a further clinical benefit in terms of the number and/or severity of infections [4]. This paper will provide an overview on pathogenesis and surgical treatment of recurrent acute tonsillitis (RAT) in children (Section 1) and it will review the therapeutic role of (adeno)tonsillectomy such patients to draw practical recommendations for clinical practice (Section 2).
Pharyngeal pain may be due to infection of the pharynx and/or tonsils but, as there are no specific diagnostic criteria, it is difficult (if not impossible) to distinguish pharyngitis and tonsillitis in clinical practice and the literature often refers generically to “sore throat” [4]. Since the publication of the paper by Paradise et al. in the New England Journal of Medicine [5], many authors have differentiated tonsillar infections into severe, moderate or mild, but without specifying whether they are bacterial or viral, and so neither their site nor their etiology is defined. Nowadays, most authors define episodes of acute purulent tonsillitis as those that are medically documented as being characterised by the presence of pyrexia (>38.3 °C), tonsillary exudate, and jugodigastric adenopathy, and requiring antibiotic treatment [5,6].
Furthermore, the nosological terms “recurrent acute tonsillitis” (RAT) and “chronic tonsillitis” (CT) are usually used as synonyms, thus making it difficult to compare different trials. Burton [4] has recently defined RAT as the presence of at least two acute episodes in the preceding 12 months, and CT as the presence of persistent symptoms lasting more than three months; however, most authors define severe RAT as five episodes of acute tonsillitis a year, the presence of symptoms for at least a year, disabling episodes and limitations on everyday activities [7], whereas others continue to use Paradise’s definition of at least five episodes a year in the two preceding years, or at least three a year in the three preceding years [5,6]. It should also be pointed out that, unlike in the case of acute rhinosinusitis, the term “CT” or “CT with recurrent acute exacerbations”, which suggest the presence of chronic structural changes in tonsillar tissue with periodic acute episodes [8], should be avoided because of the absence of real chronic tonsillar inflammation associated with symptoms lasting for more than four weeks and permanent structural alterations [3].
Most episodes of acute tonsillitis are caused by viral infections (i.e. adenovirus, Epstein- Barr virus, bocaviruses, influenza and para-influenza viruses, rhinovirus, enteroviruses including coxsackievirus, coronavirus, respiratory syncytial virus, metapneumovirus and HIV) or bacterial infections (group A betahemolytic Streptococcus Pyogenes mainly transmitted by means of Flügge droplets from infected subjects or, more rarely, healthy carriers) [1]. Other bacterial species that have been less frequently encountered include group C and G streptococci, Haemophilus influenzae, Nocardia, Coriobacteriia, Neisseria gonorrhoeae, and members of the Fusobacteria and Borrelia families responsible for Vincent’s angina, which is characterised by the onset of a unilateral ulcerous and necrotising form of tonsillitis [1]. Finally, some recent studies of children with RAT have highlighted the role played by bacterial biofilms: i.e. metabolically quiescent polymicrobial bacterial colonies inside a polysaccharide matrix that makes them extremely resistant to the action of the immune system and the administration of antibiotics [8-10].
Tonsillar surgery has always been one of the most frequent pediatric procedures and is generally performed from the age of three years [4]. It is estimated that about 530,000 pediatric procedures were performed in the United States in 2006 [11], but an analysis of the published data shows that:
a) The total number of patients undergoing tonsillar surgery has decreased because of declining support for the focal infection theory, according to which the tonsils were the starting point of the spread of pathogens responsible for the development of remote and systemic processes such as rheumatic fever, endocarditis, myocarditis, pericarditis, glomerulonephritis, pancreatitis, chorea, arthritis, and even appendicitis and peptic ulcers [12].
b) The number of children undergoing traditional tonsillectomy in Europe has also decreased because of the greater use of alternative surgical techniques, including volume reduction [3,12]; a recent review by Stelter (2014) has highlighted the fact that 8,400 of the 68,800 patients undergoing tonsillar surgery every year undergo partial tonsillectomy [3].
c) Regardless of the technique used, most authors agree that the ability of tonsillectomy to reduce the number of episodes of RAT is limited to the first 12 post-operative months [3-6,13- 16].
This refers to the complete removal of tonsillar tissue, which may be done using ‘hot’ electro-surgical instruments (mono- or bipolar electro cauters, lasers or radiofrequency instruments) for both dissection and hemostasis, or ‘cold’ dissection with a scalpel followed by electro-surgical hemostasis [17-21]. ET generally involves identifying the larger calibre afferent vessels during dissection and closing them outside the capsule by means of sutures or electro-surgical methods. This means that, regardless of the technique used, the peri-tonsillar pharyngeal musculature undergoes a substantial trauma that may expose the adjacent nervous and vascular structures to direct contact with saliva, thus leading to a bacterial super-infection that favours the development of some of the main morbidities associated with the procedure such as post-operative pain and bleeding (4.5% of cases) [12]. Furthermore, the findings of some meta-analyses suggest that ‘cold’ techniques are associated with an increased risk of intraand early post-operative bleeding (i.e. within 24 hours according to the classification of Sarny et al.) [22], whereas ‘hot’ techniques are more subject to the risk of delayed post-operative bleeding (i.e. after >24 hours) [22] and severe post-operative pain [23,24].
This procedure, which can be performed using many kinds of instruments (a CO2 laser, ultrasound scalpel, hot knife, bipolar scissors, bipolar radiofrequency coblator, microdebrider, or monopolar argon laser) [25-29], reduces the volume of tonsillar tissue medially up to the palatine arches, thus allowing the removal of the excess tissue extending into the oropharyngeal cavity while preserving the residual and immunologically active lateral tissue. It has been reported that, in comparison with ET, this procedure reduces post-operative pain and the risk of post-operative bleeding [30,31] insofar as the extra-capsular vascular and nervous afferents are not exposed.
The methods described above can also be used for an intracapsular tonsillectomy (the sub-total [90%] removal of the tonsillar parenchyma) in which the piecemeal dissection medially reaches the tonsillar capsule without going beyond it but leaves it in a tonsillar cavity covered by intact muscle tissue [32-35]. Like TT (and for the same reason), this procedure generally leads to a lower risk of post-operative bleeding and pain than ET [33], but there are no specific indications for pediatric IT because ET is preferable in patients with RAT, and TT is preferable in those with obstructive hyperplasia.
Pertinent studies published until December 2017 were selected on January 2018 by means of a MEDLINE search (accessed via PubMed) using the following terms: “tonsillectomy and children and recurrent acute tonsillitis”. We only considered fully accessible English language papers published in peer-reviewed journals and dealing with surgical management of TAR in children. Randomizedcontrolled studies, metanalysis and systematic reviews were selected. The reference lists were subsequently reviewed to ensure that all of the selected papers were truly relevant and identify any possibly overlooked and pertinent papers. Analysis of the literature was performed in order to separately evaluate the main efficacy outcomes (i.e. number and severity of episodes; number of days of pharyngeal pain; number of lost school days; tonsillectomy vs. adenotonsillectomy; long-term efficacy of surgery; surgery-related morbidity; comparison of instrumentation; impact of tonsillotomy and comparable reducing techniques on pediatric RAT); when there was a sufficient number of published studies, the results will be analysed using the GRADE Working Group’s system of defining the overall quality of the evidence provided by the meta-analyses (Table 1) [36,37]. The quality of the evidence indicates the degree of certainty with which the correctness of an estimated effect can be accepted, and the parameter can be used to interpret the results [36,37].
15 out of 96 initially selected papers were considered for literature analysis. They included six randomized controlled trials. Evaluating effectiveness of surgical treatment in children with RAT it needs to be remembered that there is generally a discrepancy between the degree of subjective satisfaction expressed by the patients’ parents and the objective clinical benefit of surgery as measured by means of standard parameters such as the number of episodes or the number of days of pharyngeal pain and their severity. It has recently been pointed out by some authors that, in addition to these classic criteria, it is also necessary to consider additional parameters that reflect the quality of life of both the patients and their families [38], such as psycho-social and educational factors, and general (fever, asthenia, nausea, etc.) and local symptoms (a sensation of pharyngeal burning, difficulties in swallowing, halitosis, cough, speaking difficulties, etc.). An analysis of the literature shows that the efficacy of tonsillectomy is limited to the 12 months following surgery and is mainly judged based on the reduction in the number of episodes and the severity of the symptoms [3-6,13-14]. In the case of patients with mild symptoms (unlike those experiencing moderately severe or severe episodes), it seems that the benefits are not sufficient to balance the disadvantages of the procedure, such as its direct and indirect costs, and the morbidity and complications arising from it [2,4,5,6,12,24].
The following paragraphs discuss in detail the main efficacy outcomes.
Table 2: Summary of evidence and practical advice relating to tonsillectomy in pediatric patients with recurrent acute tonsillitis (RAT).
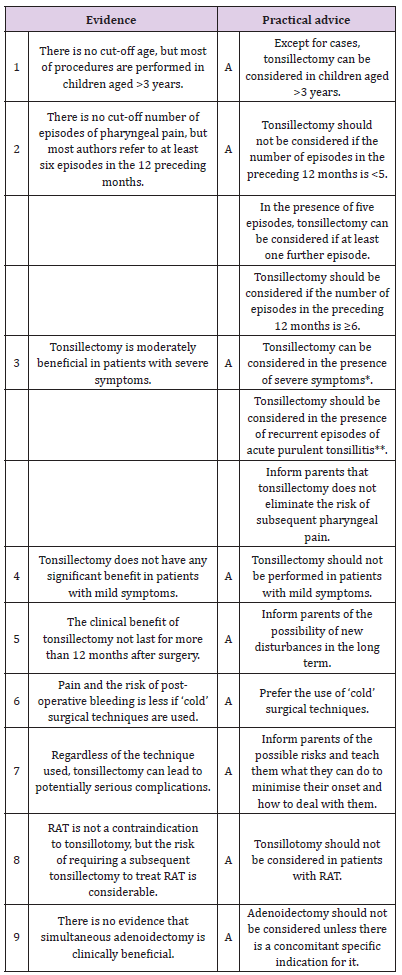
*Characterised by ≥5 episodes in the preceding 12 months, and debilitating symptoms limiting everyday activities for at least one year.
**Characterised by clinically documented tonsillar exudate, hyperpyrexia (>38.3 ºC) and jugo-digastric lymphadenopathy and requiring antibiotic therapy.
Given the extreme variability of the studies, it is not possible to draw any definite conclusions nor establish any precise threshold values concerning patient selection criteria. However, most authors agree that surgery should be avoided in the case of patients who have experienced fewer than three documented episodes of tonsillitis during their lives [2,12]. Furthermore, given the high rate of spontaneous resolution, Windfuhr believes it is advisable to observe the patient for a period of three months to determine the real need for surgical treatment [12]. Although characterised by considerable heterogeneity (especially in relation to diagnostic criteria), recent meta-analyses [4,12,24] indicate that the children undergoing surgery experience an average of 0.6% fewer episodes of pharyngeal pain of any severity during the 12 months after surgery, including the episode attributable to surgery (adenotonsillectomy or tonsillectomy vs no surgery (95% confidence interval [CI] 0.1- 1.0) than controls (five studies, 795 patients; GRADE: moderate) [4]. The patients undergoing (adeno)tonsillectomy experience 3.0 episodes (including the predictable episode of pharyngeal pain immediately after the operation) against the 3,6 episodes experienced by the children in the non-surgical group [4,12,24]. The difference is further reduced if the groups are stratified based on the severity of the episodes [4,12,24]:
a) the only available study of patients experiencing moderate or severe episodes [5] documented 1.1 episodes in the surgical group (including the predictable episode immediately after the operation) vs 1.2 episodes in the non-surgical group (difference: 0.1, 95% CI < 0.4-0.6) (one study, 73 patients; GRADE: low) [4].
b) among the patients experiencing milder episodes, the benefit of surgery is annulled (1.2 vs 0.4 moderate or severe episodes, including the predictable episode immediately after the operation): difference: 0.8, 95% CI 0.7-0.9; three studies, 491 patients; GRADE: moderate) [4].
In conclusion, although significant, the modest effect of tonsillectomy on RAT can be explained by the fact that the procedure is intrinsically characterised by pharyngeal pain and that the surgical removal of a palatine tonsil does not exclude the subsequent onset of successive episodes of pharyngitis.
Surgery reduces the number of days of pharyngeal pain during the following 12 months by an average of 5.1 days (95% CI 2.2-8.1) in comparison with no surgery (average 23.2 days, range 18.9-49.1) (five studies, 776 patients; GRADE: moderate), including those in the immediate post-operative period [4].
Children undergoing surgery miss an average of 2.3 fewer school days (95% CI 1.2-3.4) during the following 12 months because of pharyngeal pain (excluding the days immediately after surgery) than the average of six days lost by controls (four studies, 412 patients, GRADE: moderate) [4].
The only randomised clinical trial analysing this outcome [6] did not find any difference between the two approaches in terms of the number of episodes or the duration of pharyngeal pain after the operation. However, the limitations of the primary outcome and power of this study means that its conclusions may have been influenced by an underestimate due to the insufficient size of the sample [4]. Consequently, given the paucity of the number of studies, it is still not possible to say whether the addition of adenoidectomy reduces the frequency and severity of post-operative episodes of pharyngeal pain.
Most of the available studies have a high percentage of patients (about 20%) who were lost to follow-up, especially during the second and third years [3-6,13-16]. Only Stafford et al. (1986) [13] report the number of patients considered cured between 18 and 24 months after treatment: 18/20 (90%) in the surgical group and 14/20 (70%) in the non-surgical group (risk ratio 0.86, 95% CI 0.57-1.29) [4]. It is therefore not possible to say with any certainty whether surgical treatment has a significant impact on the history of RAT beyond 12 months.
None of the studies included in the meta-analysis of [4] specifies which surgery-related morbidities were evaluated other than post-operative pain, for which the studies of [5,6] document an average of 4.9-6.3 days (range 0-21). The estimated overall rate of complications is 6.0-7.9%) [5,6,39], which included early and late bleeding (respectively 1.5-4.8% and 4.3-6.0%), sometimes requiring surgical revision (including the ligation of neck vessels) or blood transfusions [4,6,12,24], nausea and vomiting (3.4%) [14], the need for hospitalisation because of bleeding or severe post-operative pain (2.1-4.3%) [4,6,12,24,40], anesthesia-related trismus, and the possible development of malignant hyperthermia (0.5%) [6]. Other complications include iatrogenic lesions of the temporo-mandibular joint and the lips, tongue, teeth and uvula; tonsillar residues requiring surgical revision; dysphonia, rhinolalia and velo-palatal insufficiency; lesions of the hypoglossal, glossopharyngeal or vagus nerve, with consequent permanent or transient dysphagia and dysgeusia; purulent cervical infection; thrombosis of the internal jugular vein; cervico-facial emphysema; mediastinitis; and Griesel’s syndrome [12] (GRADE: low) [4]. None of the studies included in the meta-analyses reported deaths among the surgical complications [4,24], but there have been some sporadic reports [41-43].
The main outcome in this regard is the comparison of ‘cold’ and ‘hot’ surgery (surgical diathermy and coblation used for dissection and hemostasis) in terms of bleeding. The main systematic reviews [4,24] indicate that electro-surgery leads to a greater risk of delayed post-operative bleeding (including cases requiring surgical revision) than cold dissection using a blade and surgical ligation (9 studies, 780 patients; GRADE: very low) [4]. The latter approach is associated with a lower overall risk of post-operative bleeding (1.7-2%), but a greater risk of intra-operative bleeding [24]. Cold surgical dissection with selective bipolar hemostasis is characterised by the lowest risk post-operative bleeding requiring surgical revision (0-0.7%) [24].
Once again, the published clinical experiences are highly heterogeneous in terms of surgical techniques and successful outcomes. However, a recent systematic review of 86 studies involving a total of 20,950 patients has found that the frequency of TT and IT is similar (respectively 43% and 41%), whereas ablative procedures (tonsillar ablation using radiofrequency, laser or coblator) account for 16% [44]. A microdebrider is the most frequently used instrument, but it is still not possible to draw any definite conclusions concerning its advantages over others. About the indications for surgery, TT is generally used to reduce the volume of tonsils obstructing the upper airways in patients with RAT, which most authors do not consider a contraindication to TT [45-47]. Given the lack of specific information in more than half of the studies, it is not possible to make a comparative metaanalysis of the different approaches in terms of the duration of the procedure, post-operative pharyngeal pain or tonsillar regrowth. The only thing that can be said is that partial or sub-total removal takes less time than traditional tonsillectomy, and causes less intra-operative bleeding, and post-operative pain and dysphagia [31]. TT is characterised by a 0.26% (range 0-2.5%) risk of postoperative bleeding requiring surgical revision, and a level of postoperative pain of 2-3 on a 0-10 visual analogue scale (VAS) whereas a traditional tonsillectomy is associated with an average VAS score of 5 [3,12,20,29,48,49]. On the other hand, the median risk of post- TT tonsillar regrowth and RAT are respectively 3.0% (0-26.9%) and 3.9% (0-16.3), and tonsillectomy is necessary to correct the regrowth or RAT in a median of 1.5% of cases (0-11.9%) [12].
There is still no published consensus concerning the age-related number of previous episodes of acute purulent tonsillitis associated with pyrexia (>38.3 ºC), tonsillar exudate and jugodigastric lymphadenopathy, and requiring antibiotic treatment, that identifies candidates for surgery (Table 2). Furthermore, the high rate of spontaneous resolution suggests the appropriateness of a six-month period of observation to verify the real need for surgical treatment (Table 2).
An analysis of the literature indicates that the efficacy of (adeno)tonsillectomy in treating pediatric RAT is generally limited and relatively transient [4,12,24].
a) Children with moderate or severe episodes (according to the criteria of Paradise et al.) [5] who undergo surgery generally experience a significant but predictable period of pharyngeal pain immediately after surgery and a further two episodes in the subsequent 12 months (1 predictable + 2 unpredictable episodes = 3 episodes in total) against an average of 3.6 unpredictable episodes in conservatively treated patients; however, depending on the case series, this average difference of 0.6 episodes may vary from 0.1 to 1.0 episodes, and so there may be no real difference between the two groups.
b) In children with milder episodes, the reduction in the risk of developing subsequent moderate or severe episodes because of surgery is even less (1 predictable + 0.2 unpredictable episodes = 1.2 episodes in total vs 0.4 unpredictable episodes in conservatively treated children).
c) Children undergoing surgery experience an average of 18- 23 days of pharyngeal pain in the subsequent 12 months, but these include the 5-7 days following the operation.
d) The clinical benefit of surgical treatment is only apparent during the first 12 post-operative months.
Although tonsillectomy seems to have a positive impact on the quality of life, analysis of the literature reveals that there is an urgent need for controlled studies using standardised and reproducible parameters. Based on the currently available data, it is advisable in clinical practice to inform the parents of children with RAT who are candidates for tonsillectomy that, although significant, the clinical benefit in terms of the reduction in the number of episodes of pharyngeal pain may be limited in the presence of severe symptoms and non-existent in the case of mild symptoms, for which non-surgical treatment should be considered (Table 2). Cold tonsillectomy seems to be the technique that is least burdened by pain and the risk of post-operative bleeding, but also seems to be characterised by a slight increase in the risk of intra-operative bleeding. On the contrary, the use of electro-surgery is associated with a lower risk of early bleeding, but an increased risk of delayed post-operative bleeding (and estimated overall bleeding), and so its use in clinical practice needs to be carefully weighed against its potential disadvantages and, if possible, it should be avoided (Table 2).
Although a lack of data and the absence of appropriate followup in a considerable number of case series makes it impossible to reach a sufficient level of evidence to indicate the superiority of TT, the available data suggest that it and comparable procedures are better tolerated than traditional tonsillectomy in terms of the duration of surgery, intra-operative bleeding, the resumption of oral feeding, pain, and post-operative complications. Analysis of the literature also shows that it is generally less frequently performed than tonsillectomy, and none of the guidelines include it among the therapeutic strategies for patients with RAT [12] except for the recent German guidelines, which mention it as a possible alternative to traditional tonsillectomy in patients who have experienced at least six episodes in the preceding 12 months or 3-5 episodes after a 6-month observation period during which it is mandatory to reach a total of at least six episodes, regardless of the degree of tonsillar hypertrophy (which must in any case be >1 according to Brodsky’s classification). This position is supported by the fact that the available evidence suggests that TT resolves upper airway obstruction and improves the natural history of patients with RAT, and the fact that there are still no studies proving the superiority of tonsillectomy over volume reduction in such patients. However, although not generally very high, the risks of tonsillar regrowth, the onset of RAT requiring surgical revision, and subsequent tonsillectomy have respectively reached 27%, 16% and 12% in some case series [12], and so the decision to perform TT in a patient with a history of RAT should be made very cautiously (Table 2). Finally, there is currently no evidence to suggest that simultaneous adenoidectomy and tonsillar surgery is clinically beneficial in patients with RAT, and so adenotonsillectomy should only be performed in the presence of specific conditions (Table 2).


