Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Renata Zawisza*1, Leszek R Jaroszewicz1, Anna Celebańska2, Predrag Mikulic2 and Wojtek J Bock2
Received: October 09, 2018; Published: October 29, 2018
*Corresponding author: Renata Zawisza, Institute of Applied Physics, Military University of Technology, Poland
DOI: 10.26717/BJSTR.2018.10.001967
A dual-resonance long period grating (DR-LPGJ embedded in fiber loop mirror (FLM) was employed as a sample for the development of the biosensor application. The DR-LPGJ acts as a sensing part for biological molecules detection, whereas FLM enhances the rapid signal monitoring, which oversees the wavelength filtering properties. Herein, the DR-LPG surface was functionalized with biotin in terms of the avidin binding reaction detection. The detection mechanism is based on the alteration the refractive index of the DR-LPG surrounding monitored by the tracking interference dips changing. Moreover, by applying a broadband diode and photodetector we ensured the low-cost of the system.
Abbreviations: DR-LPG: Dual- Resonance Long Period Grating; FLM: Fiber Loop Mirror; LPG: Long Period Grating; RI: Refractive Index; APTES: Amino Propyl Tri Ethoxy Silane; EDC: 1-Ethyl-3-(3-DimethylaminopropylJCarbodiimide; PBS: Phosphate Buffer Saline; LOF: Lab-On-a-Fiber
An optical fiber based sensor are very useful in many applications because of the compact size, electrical interference independence and suitability for remote sensing [1]. Recently, a significant effort has been devoted for the long period grating (LPGJ - based sensors for medical application [2,3]. The real-time detection, high sensitivity and accuracy are desire properties for the medical sensors in terms of the facilitate prompt disease diagnosis [3]. In spite of the high sensitivity of the LPG, the main asset is possibilities of modifying the optical structure with thin functional coatings in terms of facilitating highly selective and fast measurement in real time with the reported resolution of refractive index (RIJ change up to 10-6 [4]. To detect any molecular elements, the LPG surface needs to be functionalized by e. g. enzymes [5], gold nanoparticles [6], antibodies [7] or cross- linking bioreceptors [8]. The LPG is an optical periodic structure, where transmission spectrum consists of the resonance dips at discrete wavelength [9]. When it comes to achieve very sensitive structure, the LPG should work close to the turning point, where the dual-resonance appears.
Such dual- resonance long period grating (DR-LPGJ has been shown to be ultra-sensitive [10] regarding the micro-scale label- free detection [11]. The detailed theory of the DR-LPG structure can be found in [12,13]. To satisfy the need for very accuracy molecules detection and to maintain a stable temperature of the measurement, the DR-LPG can be combining with other optical elements [14]. Among various solution, the fiber loop mirror (FLMJ provides several advantages, including high extinction ratio, and low cost of final arrangement. The part of high birefringence fiber (Hi-Bi fiber] from FLM structure ensures ambient temperature changes controlling by the interference dips shifting/amplitude intensity changing observation. Moreover due to filtering properties of the FLM, it provides possibilities of control of the wavelength spacing between interference dips, limitation of the temperature and strain cross- sensitivities and consequently enhancement of the sensitivity of the specific sample detection [15].In this paper, for the first time of our knowledge, we present the combined structure of the DR-LPG in the FLM for biotin-avidin reaction as a potential platform for medical application.
In particular, the described optical platform can be applied as a sensor for limitless biomarkers or molecules, similarly to LPG which was already used to detect, e.g. bacteria [16]. It is possible due to the ultra-high sensitivity towards a refractive index of surrounding environment. Moreover, the introduction of a reception layer to the sensing system ensures its selectivity exclusively towards the desired analyte.
Here, we present a proof of concept of the application of our sensing platform by applying the well-known biological model, biotin-avidin complex. Esmond Snell discovered the biotin- avidin interaction during the investigation on "egg-white injury" disorder in 1941 [17]. Biotin calls also vitamin H, it is an crucial compound taking part in the metabolism of fatty acids and amino acids. The protein, avidin from raw egg white has capability to bind biotin causing its deficiency in mammalian bodies. The avidin- biotin binding belongs to the most robust noncovalent biological interaction with a dissociation constant 10-15 [18]. In the presented system, biotin is applied as a receptor, immobilized covalently to the surface, able to selectively capture of avidin dissolved in the solution.
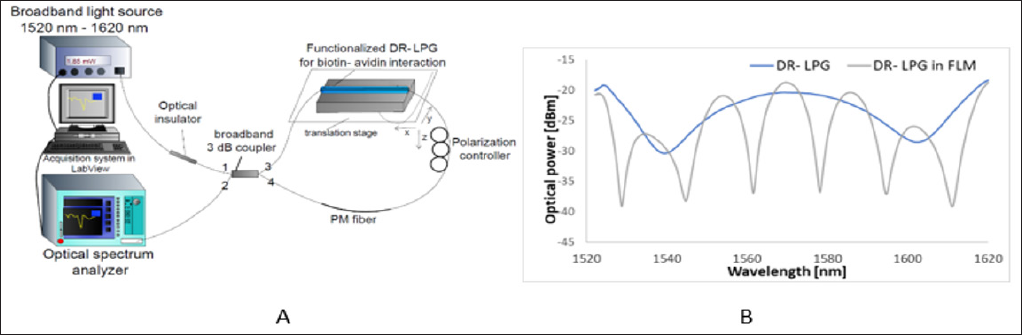
The DR-LPG was produced using amplitude mask technique, where the part of bare fiber was exposed on UV-radiation using KrF Excimer laser (Lumonics™ Lasers: Pulse Master ®-840) emitting at 248nm. The period of the used DR-LPG was 217 μm. The experimental platform used for monitoring biotin-avidin reaction is shown in Figure 1a and has been in details described in [19]. The transmission spectra of proposed platform are shown in Figure 1b, where the DR-LPG in FLM spectrum was adjust by a polarization controller in terms of maximum set-up response.
Figure 1: The scheme of the experimental platform for biotin-avidin interaction (a) and transmission spectra of DR-LPG (blue line) and DR-LPG in FLM (grey line) (b).
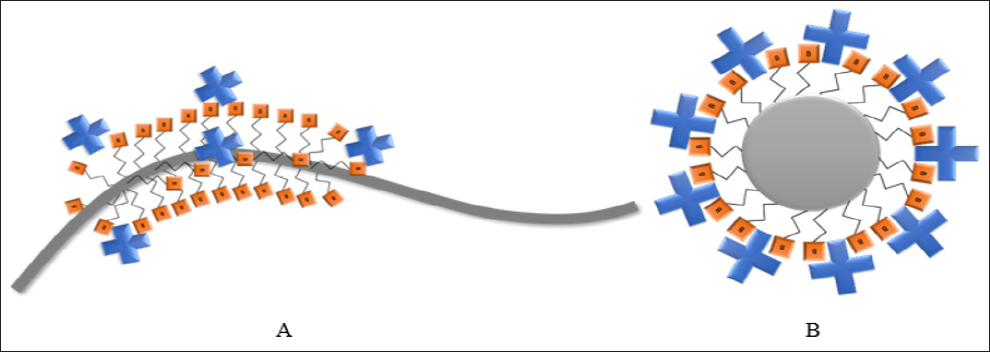
The DR-LPG surface was cleaned from organic impurities by soaking in hydrochloric acid (aq.)/methanol (1:1, v/v) mixture and next in concentrated sulfuric acid for 30 min, followed by plentiful rinsing with water. After that, the platform was dried in vacuum for 15 min and transferred for the aminization process. The DR-LPG was locked into the desiccator chamber together with trays of 30 μl of 3-aminopropyltriethoxysilane (APTES) and 10 μl of triethylamine for 30 min and/or 2h in the argon atmosphere [20]. In the next step, the biotins carboxylic group was activated by EDC/NHS to form an amide bond with amine groups of the APTES covered surface. For this, the biotin powder was dissolved in the mixture of water and dimethylformamide (1:1, v/v) containing 0.8 M 1-ethyl-3- (3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) and 60 mM N-hydroxysuccinimide (NHS). The final concentration of biotin was equal 1 mg ml-1. Next, the APTES covered platform was immersed in the prepared solution for 1 hour in a sealed chamber. After this time, DR-LPG was rinsed with water and exposed to avidin for 30 min. Avidin solution was prepared by it dissolving in phosphate buffer saline (PBS) pH = 7.4 to obtain concentration equal 1 mg ml-1. Figure 2 shows the scheme of functionalised DR- LPG surface with a biotin-avidin interaction.
The DR-LPG surface was cleaned from organic impurities by soaking in hydrochloric acid (aq.)/methanol (1:1, v/v) mixture and next in concentrated sulfuric acid for 30 min, followed by plentiful rinsing with water. After that, the platform was dried in vacuum for 15 min and transferred for the aminization process. The DR-LPG was locked into the desiccator chamber together with trays of 30 μl of 3-aminopropyltriethoxysilane (APTES) and 10 μl of triethylamine for 30 min and/or 2h in the argon atmosphere [20]. In the next step, the biotins carboxylic group was activated by EDC/NHS to form an amide bond with amine groups of the APTES covered surface. For this, the biotin powder was dissolved in the mixture of water and dimethylformamide (1:1, v/v) containing 0.8 M 1-ethyl-3- (3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) and 60 mM N-hydroxysuccinimide (NHS). The final concentration of biotin was equal 1 mg ml-1. Next, the APTES covered platform was immersed in the prepared solution for 1 hour in a sealed chamber. After this time, DR-LPG was rinsed with water and exposed to avidin for 30 min. Avidin solution was prepared by it dissolving in phosphate buffer saline (PBS) pH = 7.4 to obtain concentration equal 1 mg ml-1. Figure 2 shows the scheme of functionalised DR- LPG surface with a biotin-avidin interaction.
Figure 2: The scheme of the DR-LPG functionalised with a biotin (orange square) interacting with avidin (blue cross) from the solution: a) the whole platform, b) the cross-section of the platform.
All presented measurement data was performed in a fresh PBS imitating human blood serum, and every step of functionalization process was followed by the DR-LPG excessive wash to remove weakly bonded biomolecules. This procedure allowed to obtain a reliable response. Moreover, to maintain stable physical conditions the temperature was monitored, and the DR-LPG was kept in a constant volume of PBS. The introduction of the sensor into a narrow tube allowed to control and suppressed unwanted solution evaporation. The spectrum was recorded three times in PBS. Firstly, after the surface sensor silanization process (Figure 3J, light blue lineJ. Secondly, after the biotin covalently attachment to the sensor (dark blue lineJ. And thirdly, afterword the sensor interaction with avidin (grey lineJ. The red dashed lines in Figure 3 mark the notches of the DR-LPG which corresponds with those from Figure 1b and only these minima are considered in regards of sensing properties of the device. When the ambient refractive index varying, the DR- LPG notches (around 1530 nm and 1615 nmJ shifting toward each other, and hence, the amplitude of the interference dips of the DR- LPG in FLM is changed.
Figure 3: The experimental platform response for DR-LPG surface silanization (light blue line), DR- LPG immersed on PBS after biotin deposition (dark blue line) and DR- LPG immersed n PBS after avidin deposition (grey line). The left/right magnifications show 1530 nm and 1615 nm region, respectively.
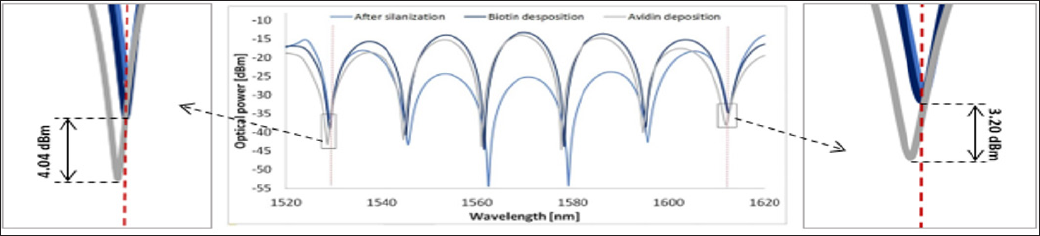
The fact, that amplitude of the transmission spectrum of the DR-LPG in FLM significantly increase after biotin disposition means that biotin was stable coated to the DR- LPG surface and BSA could not rinse it from the surface. As can be seen from Figure 3, only the amplitude of the covered by the DR-LPG notches interference dips decrease after avidin attachment. The observed change is estimated to 4.04 dBm and 3.20 dBm, respectively to 1530 nm and 1615 nm. The experiment confirmed that presented platform combined with appropriate receptor can be applied as a sensor for biological analytes detection.
We presented a potential application of the DR-LPG in FLM platform for label-free biochemical detection. The proposed solution fits squarely into technology named Lab-On-a-Fiber (LOFJ (more specifically Lab-around-FiberJ, which is dedicated to devices focused on the design and development of advanced fiber optic nanoprobes for biological applications. Hence, the LOF enables continuous monitor of detected samples. Adapting FLM in the sensor probe (DR-LPGJ influences on avoiding thermal and strain cross- sensitives, and this limitation dealing with pure unconventional light matter interaction in confined small-scale volumes. Despite of many asset of the FLM configuration, it ensures relatively high sensitivity due to the phase shift keying. Finally, throughout adoption of the laser diode, (broadband light source) photodetector and the commercially available optical passive elements with the telecommunication standards, the DR-LPG in the FLM configuration provides the low- cost usable sensor implementation. Therefore, we can conclude that proposed platform can monitor the biotin- avidin interaction and is good candidate to some other biological sample detection as a medical application.
This research was funded by the financial support of the Natural Sciences and Engineering Research Council of Canada for the SPI/NSERC Industrial Research Chair in Photonic Sensing Systems for Safety and Security Monitoring. The investigation was also supported by the internal MUT project No. RMN 08/690 as well as project under the Ministry of National Defense Republic of Poland Program-Research Grant MUT project No. 13-995.


