Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Masashi Sakuma1,3, Satoko Kishimoto2,3, Ken-ichi Inoue2,3, Ryoichi Sohma2, Shigeru Toyoda1,3, Hideki Iwaguro1,3 and Teruo Inoue*1,2,3
Received: December 12, 2018; Published: December 21, 2018
*Corresponding author: Teruo Inoue, Department of Cardiovascular Medicine, Japan and Satoko Kishimoto, Research Support Center, Center for Regenerative Medicine Tochigi, Japan
DOI: 10.26717/BJSTR.2018.12.002265
Background: Low molecular weight heparin/protamine micro/nanoparticles (LH/P-MPs) has been shown as cell carriers for adipose-derived regenerative cells (ADRCs) to augment cell viability, leading to accelerating angiogenesis in mouse limb ischemia models, compared to ADRCs alone.
Material & Methods: Human ADRCs were isolated from 3 patients with critical limb ischemia and incubated with and without LH/P-MPs Incubation of ADRCs with LH/P-MPs produced cell aggregation (ADRC/LH/P-MPs aggregates). Serial changes in survived ADRCs were compared between ADRC/LH/P-MPs aggregates and ADRCs alone.
Results: Patients' derived ADRCs promptly decreased cell number in suspension culture, eventually they were eliminated through cell death after seven days. ADRC/LH/P-MPs aggregates remarkably slowed down the cell death. Conclusion: The LH/P-MPs could enhance the viability of human ADRCs. The LH/P-MPs could be a promising option in ADRCs-based therapeutic angiogenesis in patients with critical limb ischemia.
Keywords: Limb Ischemia; Adipose-Derived Regenerative Cells; Low Molecular Weight Heparin/Protamine Micro/Nanoparticles; Angiogenesis Therapy; Human
Recently stem cell therapies have been widely applied for various diseases refractory against conventional medical as well as surgical treatments. Critical limb ischemia due to peripheral artery disease or Bürger disease is a rational indication for the stem cell implantation to induce neovascularization. Bone marrow-derived stem cells (BMSCs) can improve limb ischemia after injection in experimental limb ischemia models [1] and patients with critical limb ischemia [2]. Mesenchymal stem cells (MSCs) are also known to induce neovascularization in a hindlimb ischemia model [3]. Adipose tissue is abundant in the human body and is constantly reorganized through angiogenesis. Therefore, this tissue is an ideal source of angiogenic MSCs. It has been shown that adipose-derived regenerative cells (ADRCs), which contain not only MSCs but also various regenerative cells such as endothelial progenitor cells, have characteristics similar to those of BMSCs [4]. The implantation of in vitro cultured ADRCs in the mouse hindlimb ischemia model was effective for its proangiogenic action [5]. In addition, the safety and effectiveness of autologous ADRCs implantation in patients with critical limb ischemia has been reported [6].
Recently an automated cell-processing system for the isolation of ADRCs has been developed and its safety and reproducibility have been assessed [7]. We have recently reported low molecular weight heparin/protamine micro/nanoparticles (LH/P-MPs) can be used as cell carriers for autologous ADRCs in mouse hindlimb ischemia model. The LH/P-MPs bind to the cell surface of ADRCs through heparin-binding proteins such as integrins. The interaction of the ADRCs with LH/P-MPs results in the formation of ADRC/ LH/P-MP aggregates, sustaining survival of the cells. In the inbred mouse hindlimb ischemia model, these aggregates efficiently increase cell viability in vitro and injection of them augmented neovascularization in vivo, compared to ADRCs alone. As a result, the ADRC/LH/P-MP aggregates were more effective in preventing the loss of ischemic hindlimbs compared with ADRCs alone [8]. However, the superior effect of ADRC/LH/P-MP aggregates on ameliorating critical limb ischemia over ADRCs alone has not been confirmed in human. In the present study, we investigated whether the LH/P-MPs augment cell viability in human ADRCs in vitro.
ADRCs were isolated from periumbilical subcutaneous adipose tissues collected by a liposuction procedure in 3 patients with critical limb ischemia (Case 1: 51yo male; progressive systemic sclerosis, Case 2: 70 yo female; progressive systemic sclerosis and Case 3: 45yo male; Bürger disease), who were planned to undergo the stem cell therapy for ischemic limbs. For the isolation of ADRCs, we used an automated cell-processing system, Cytori Celution device (Cytori Therapeutics Inc., San Diego, California). The stem cell therapy was performed under the approval of local committee for regenerative medicine and an informed consent regarding the present study was obtained from each patient. The LH/P-MPs were synthesized as described previously [9]. Briefly, 0.3mL of protamine sulfate solution (10mg/mL; Mochida Pharmaceutical Co., Tokyo, Japan) was added dropwise to 0.7mL of a low-molecular-weight heparin solution, Dalteparin sodium (6.4 mg/mL; Kissei Pharmaceutical Co., Tokyo, Japan) and vortexed for approximately 2min. The mixed LH/P-MPs were then washed twice with phosphate-buffered saline to remove nonreactants using centrifugation at 4,900g for 5min, and the precipitates were finally resuspended in 1mL of Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies Oriental, Tokyo, Japan).
Subsequently, more than 6mg of dry LH/P-MPs were obtained from 1mL of the resuspended LH/P-MPs solution. In the final preparation step, 60mg of LH/P-MPs were resuspended in 1mL of DMEM. ADRCs isolated from each patient were incubated in DMEM containing heat-inactivated fetal bovine serum with and without LH/P-MPs (about 1.4mg of dried particles/mL) in 15mL polypropylene conical tubes with occasional shaking at 37 ℃ for the indicated time periods. Incubation of ADRCs with LH/P-MPs produced cell aggregation (ADRC/LH/P-MPs aggregates). Survived ADRCs were counted at the next day, and 2 days, 3 days and 7 days after the first day of incubation for 5×103 cells by the 12 experiments in each sample of the 3 patients. Statistical analyses were carried out using SPSS software (IBM, Armonk, NY, USA). To detect the main effect by LH/P-MPs, we adopted Fisher’s least significant difference procedure (analysis of variance and student’s t test as a post hoc). P value less than 0.01 were considered significant.
Patients’ derived ADRCs promptly decreased cell number in suspension culture, eventually they were eliminated through cell death after seven days. Addition of LH/P-MPs produced aggregation with ADRCs, remarkably slowed down the cell death. Such a protection effect against cell death was reproduced in three independent patients. The number of survived ADRC was significantly higher in ADRC/LH/P-MPs aggregates compared to ADRCs alone at the next day (Case 1: 4.53±0.34 vs 1.49±0.29 ×103, P< 0.01; Case 2: 4.10±0.44 vs 1.28±0.39 ×103, P< 0.01; and Case 3: 4.33±0.35 vs 1.83±0.56 ×103, P< 0.01), at the 2 days (Case 1: 4.11±0.38 vs 1.00±0.28 ×103, P< 0.01; Case 2: 3.38±0.37 vs 0.75±0.26 ×103, P< 0.01; and Case 3: 3.75±0.41 vs 1.04±0.48 ×103, P< 0.01) and at the 3 days (Case 1: 3.13±0.37 vs 0.62±0.26 ×103, P< 0.01; Case 2: 2.45±0.30 vs 0.40±0.19 ×103, P< 0.01; and Case 3: 2.72±0.50 vs 0.51±0.26 ×103, P< 0.01) (Figure 1).
Figure 1: Serial changes in viability of ADRCs. Patients’ derived ADRCs promptly decreased cell number in suspension culture, eventually they were eliminated through cell death after seven days. Addition of LH/P-MPs produced aggregation with ADRCs, remarkably slowed down the cell death. Such a protection effect against cell death was reproduced in three independent patients. ADRC, adipose-derived regenerative cell; LH/P-MPs, low molecular weight heparin/protamine micro/nanoparticles **P<0.01.
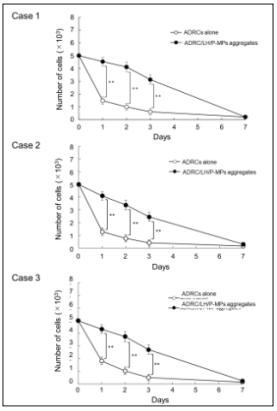
In the present study, we observed that cell viability of ADRCs isolated from human subcutaneous adipose tissues was augmented, when the LH/P-MPs were added to the ADRCs and ADRC/LH/PMPs aggregates were formed, compared with the ADRCs alone in all of 3 patients with critical limb ischemia. Survived cell number was reduced to approximately 30% at the next day, 20% at the 2 days and only 10% at the 3 days after incubation in case of ADRCs alone, indicating that ADRCs hardly sustain cell number due to lack of extracellular scaffold. On the other hand, addition of LH-P-MPs rescued such a rapid cell death and the ratio increased to 90% (next day), 80% (2 days) and 70% (3 days), respectively. The ADRCs have the potential to differentiate into various cell lineages, including vascular cells [10]. In addition, cultured ADRCs secrete various angiogenic growth factors, such as basic fibroblast growth factor, hepatocyte growth factor, and vascular endothelial growth factor as well as cytokines [11-13]. Angiogenetic mechanism of stem cell therapy such as ADRCs therapy is mainly based on paracrine effects by these angiogenic factors [14].
Apart from the protection effects as a cellular scaffold, LH/PMPs can immobilize and stabilize various angiogenic factors through heparin binding motifs. As a results, the beneficial effects of LH/P-MPs are two-fold: Sustaining survival of ADRCs per se and stabilization of various angiogenic factors or cytokines. Therefore, the precise mechanisms of augmenting angiogenesis are suggested to be as follows. First, injected ADRCs are protected from cell death by LH/P-MPs and continuously release of multiple angiogenic factors. Next, these angiogenic factors are adsorbed onto LH/PMPs, permitting their stabilization and bioavailability. Such dual effects are considered to be critical for augmented angiogenesis in vivo [8]. In the present study, we demonstrated that the LH/P-MPs could enhance the survival of patients’ derived ADRCs. Both lowmolecular- weight heparin and protamine are agents available for clinical use and the safety concern using LH/P-MPs is relatively low. Therefore, LH/P-MPs could be a promising option in therapeutic angiogenesis as cellular scaffolds for ADRCs in human.


