Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Phongsaran Kimawaha1,3, Wassana Jamnongkan2,3, Malinee Thanee2,3, Watcharin Loilome2,3 and Anchalee Techasen1,3*
Received: December 11, 2019; Published: January 02, 2019
*Corresponding author: Anchalee Techasen, Department of Clinical Microbiology, Faculty of Associated Medical Sciences, Thailand
DOI: 10.26717/BJSTR.2019.12.002284
Cholangiocarcinoma (CCA) is the cancer of bile duct which is the common cancer and the major public health problem of northeastern region, Thailand. The major cause of CCA is chronic inflammation induced by liver fluke Opisthorchis viverrini (Ov) infection. Furthermore, one of key mechanism in the development of CCA is epithelial mesenchymal transition (EMT), which is characterized by loss of epithelial markers and gain of mesenchymal markers. One of the key EMT protein is Twist, an EMT transcription regulator, provides a significant prognostic marker that could be used as an EMT molecular target. Nowadays, there is no effective CCA treatment available. Several studies have attempted to use effective natural products for prevention and treatment of cancer including CCA. In this study aims to evaluate whether xanthohumol (XN) in combination with praziquantel, anthelmintic drug effect on inflammatory and Twist expression in cholangiocarcinogenesis in hamster model. Herein, four study groups of hamsters were conducted including;
a) OV and NDMA treated (ON),
b) ON with praziquantel receiving (ONP),
c) ON with xanthohumol receiving (XON) and
d) ON combination with praziquantel and xanthohumol receiving (XONP) at 60, 90, 120 and 180 days.
The results revealed that inflammatory cells around bile ducts epithelial were decreased in ONP and XONP treated when compared with ON-treated group. However, decreasing of inflammatory cells was not seen in XON-treated hamsters. In addition, higher Twist expression was observed in ON group and the strongest staining of Twist was found at day 180. Interestingly, the reduction of Twist was seen in XON and ONP groups at day 90 when compared with the ON group. Moreover, our results exhibited an effective reduction of Twist expression around bile duct area for the XONP group. In summary, XN alone could not reduce inflammatory lesions around bile duct epithelial cells but in combination with PZQ can effectively decrease of inflammatory cell in liver. For EMT, XN and/or PZQ could potentially reduce Twist expression. Further studies are needed to confirm in depth the association between Twist and CCA prevention.
Keywords: Cholangiocarcinoma; Epithelial Mesenchymal Transition; Xanthohumol; Praziquantel; Twist
Abbrevations: CCA: Cholangiocarcinoma; OV: Opisthorchis viverrini; EMT: Epithelial Mesenchymal Transition; BHLH: Human Basic Loop Helix; PZQ: Praziquantel; NDMA: N-Nitrosodimethylamine; DAB: Diaminobenzidine Tetrahydrocholoride; IASL: International Association for the Study of the Liver; H&E: Hematoxylin and Eosin
Cholangiocarcinoma (CCA) is the most common liver cancer that has frequently incidence especially in the Northeast of Thailand. This malignant is due to the infection of liver fluke Opisthorchis viverrini (Ov), which causes cancer progression through inflammatory process around the bile duct epithelium [1]. There is a large number of free radicals resulted in oxidative stress that has the potential to destroy the biomolecules for instance DNA, lipids and proteins, as well as the genetic alteration [2]. Furthermore, it is also found that liver fluke antigens can be diffused and attached into liver cells, bile duct epithelium and found along with the inflammatory cells occurred at the area of parasite infection [3]. Liver fluke infection can cause the pathophysiology in the liver and bile ducts with various mechanisms, a sucker of living parasite can generate the abrasion in bile duct epithelium, the secretory from the parasite which is considered for antigen be able to trigger the immune response and the inflammatory process by stimulating the immune system. Induction of inflammatory cells, such as macrophage, eosinophils and neutrophils, is also commonly found to move in areas where there is Ov infection. At the same time, these cells are stimulated to produce large amounts of free radicals.
These free radicals not only destroy parasites but they can also alter molecular biology leading to CCA genesis [4]. Another factor that contributes to CCA development is the movement of the parasite in the bile duct that can cause irritation within the bile duct cells resulting in shredded of bile duct epithelium. In combination with cell damage, free radicals cause cell death and disruption of bile duct epithelium at all times emerging the enlargement and reproduction of its cells called hyperplasia [5]. Moreover, one of key mechanism in the development of CCA is epithelial mesenchymal transition (EMT), is the process which is characterized by loss of epithelial markers and gain of mesenchymal markers that supporting cancer cell metastasis [6]. The characteristics of EMT are composed of induction of transcription factors including Twist, Snail, Slug, ZEB1 and ZEB2. The human basic-loop-helix (bHLH) Twist gene is also induces metastasis in tumor cells through the EMT process particularly in the metastasis process that Twist can facilitate intravasation [7] and invasion in various cancers for instance cervical prostate endometrial [8-10] and hepatocellular carcinoma [11]. In addition, Twist overexpression is associated with the up-regulation of mesenchymal proteins in human gastric, prostate, and hepatic cancers [12].
From our previous study showed that the high expression of Twist was significantly associated with poor prognosis of CCA patients [13]. Furthermore, the correlation between Twist and inflammation has been studied and revealed that Twist proteins is the anti-inflammatory factors and pathways in regulation of TNF-α and provide a mechanism by which type I IFNs and Axl receptors suppress inflammatory cytokine production [14]. Thus, Twist provides a significant prognostic marker that could be used as an EMT molecular target and also as a treatment efficiency marker in CCA. At present, active drug treatment for liver fluke infection is praziquantel (PZQ), a broad-spectrum anthelmintic drug, causing parasite paralysis by increasing cell membrane permeability to calcium, resulting in contraction of the musculature of the parasite and eventually paralysis and death. Moreover, PZQ also causes the parasite to become accessible to eradicate by the host’s immune response. In the treatment of CCA, the gold standard is surgical resection but is often impossible to completely resect the tumor, so there are no currently practical treatments for CCA patients.
Accumulating many studies suggests that bioactive substances from plants (phytochemicals) may be ensuring chemopreventive and chemotherapeutic agents in the treatment of several human cancers. Nowadays, medicinal plants are gaining interest in bringing to study for the treatment of various diseases, including cancer because it has a broad spectrum of action and has the potential to prevent and treat cancer effectively. Therefore, efforts are being made to find substances from natural products used in the prevention or treatment of cancer. Xanthohumol (XN; 3’ (3,3‑dimethylallyl)‑2’,4’,4‑trihydroxy‑6’‑methoxychalcone), the most abundant prenylated flavonoid (0.1‑1% of dry weight) in Hops (Humulus lupulus L.)) which used to produce beer, a major dietary source of prenylated flavonoids, where it has been found at concentrations of up to 0.96mg/l (1.95μM) [15]. This substance inhibited the growth of cancer cells and increases the enzyme activity. Recently, an increasing number of studies demonstrated the broad‑spectrum anticancer and prevention activity of XN in many types of cancer such as hepatocellular carcinoma, epithelial ovarian cancer [16-17], colon cancer prostate cancer [18,19].
Additionally, in vivo and in vitro studies revealed that XN can inhibit the proliferation and induced apoptosis of cancer cells. XN can suppress Ki‐67 expression, CD31‐positive microvessel density, NF‐κB p65 expression, and VEGF and IL‐8 levels. Taken together, these results showed that XN inhibited angiogenesis by suppressing NF‐κB activity in pancreatic cancer [20]. Recently study reported that XN dramatically suppressed the growth of tumor and induced apoptosis in CCA cells by inhibited STAT3 activation due to suppression of the Akt-NFκB signaling pathway [21]. Moreover, our previous studies have shown that XN has the potential to reduce periductal fibrosis in early stages of liver fluke infection as well as efficiently reducing fibrosis in long time treatment and found the reduction of fibrosis due to Ov infection was competently eliminated in combination with PZQ treatment [22]. Thus, it is considered that XN is one chemopreventive substance that is effective in the prevention of cancer by inhibiting all process of the carcinogenesis [23]. These data suggested that XN has a potential to become a useful new medical approach for the chemoprevention of CCA. Finally, our study aimed to investigate the effect of XN with or without PZQ on inflammation and EMT in the expression of Twist protein during CCA genesis in a hamster model.
Extraction of Ov metacercariae were achieved from naturally infected cyprinid fish which were acquired from the local market in Khon Kaen Province, northeast Thailand. The fish were chopped up and digested with pepsin-HCL, then incubated in shaking water bath at 37°C for an hour. After that, the digested fish were filtered through the sieves (1,000, 425 and 106μM respectively) and it was sedimented with 0.85% saline in a sedimentation jar. Subsequently, the metacercariae were isolated and determined under a stereo microscope. The five male Syrian golden hamsters were fed by the total number of fifty viable active Ov cysts to each by intragastric intubation.
The carcinogen, N-nitrosodimethylamine (NDMA) (Sigma- Aldrich, St. Louis MO, USA), was diluted at the concentration 12.5 ppm in distilled water. Subsequently, it was administrated daily to the assigned experiment hamster groups starting on day 30 until day 60 after the infection of Ov.
The XN was obtained from Hopsteiner, Mainberge, Germany. The preparation of XN-supplemented water was performed everyday as 1μl of stock 20 mM in 250μl of distilled water, yielding a final concentration of 20 μM or 171 mg/B.W./day in the experimented groups. The pre-treated with XN to hamsters were achieved more than 14 days before the experiment launched and the XN treatment was proceeded until animals were sacrificed at day 60, 90, 120 and 180 post-treatment.
The anthelmintic drug, PZQ (Sigma-Aldrich, St. Louis MO, USA), was diluted with a non-ionic solubilizer and emulsifier that was chemophor at 2% concentration. The oral administration of PZQ was performed by a single dose of 400mg/kg to the assigned treatment groups after day 30 of Ov infection.
The study protocol was approved by the Animal Ethics Committee of Khon Kaen University (AEKKU 23/2555). The four groups of male Syrian golden hamsters (age 6-8 weeks, 2 hamsters per group) were randomly separated into as followings: group I, Ov infection and NDMA administration (ON); group II, Ov infection and NDMA administration and PZQ treatment (ONP); groups III and IV were related to group I and II by additionally received 20μM of XN (171 mg/B.W./day) designated as XON and XONP groups, respectively. Hamsters were infected with the amount of 50 Ov metacercaria by oral inoculation which was administered with NDMA at concentration 12.5 ppm in water for 30 days and withdrawn thereafter. The preparation of XN-supplemented water was performed daily as 1μl of stock 20mM in 250μl of distilled water. The hamsters were killed at 60, 90, 120 and 180 days after treatment and their liver tissues were accumulated for further studies.
Hematoxylin and eosin (H&E) staining was used for evaluation of inflammatory cells. Firstly, hamster liver sections were deparaffinized and rehydrated with graded ethanol solution. The sections were stained with hematoxylin for 6 min, washed in distilled water for 5min, and rapidly washed in 70% acid ethanol, PH 2.5 at three times. After that washed in running tap water for 15min and stained the sections with working eosin-phloxine solution for 2min. The sections were dehydrated with stepwise increasing concentration of ethanol and xylene and mounted with mounting medium. The stained sections were examined under a light microscope. The staining of H&E can separate the components of cells by color in which hematoxylin will give blue color for the nucleus and eosin will give the pink color for the cytoplasm. The histopathological evaluation of staining was adapted using the International Association for the Study of the Liver (IASL) for inflammatory cells; Grade 1: few, Grade 2: moderate, Grade 3: numerous [24].
Immunohistochemistry was performed to determine the expressions of Twist in hamster liver tissue. Briefly, the sections were passed through graded ethanol solutions to deparaffinize and rehydrate the sections. Antigen retrieval was performed by heating the sections in a microwave oven in 10mM citrate buffer PH 6.0 for 10 minutes, while 0.3% (v/v) hydrogen peroxide was used to block endogenous peroxidase activity. Then, 10% skim milk in PBS was added to block non-specific substances in tissues. Each section was incubated with primary antibody; rabbit anti-Twist (Abcam, Cambridge, UK) at 4°C overnight. After that, sections were washed in 0.1% Tween20 in PBS and incubated with horseradish peroxidaseconjugated EnvisionTM secondary antibody (Dako, USA). The color was developed with 3, 3’ diaminobenzidine tetrahydrocholoride (DAB) substrate kit (Vector Laboratories, Ca), then counterstained with Mayer’s haematoxylin. The sections were dehydrated with stepwise increasing concentration of ethanol and mounted with permount solution. The stained sections were examined under a light microscope.
Statistical analysis was performed using SPSS software V.23.0 and Graphpad Prism V.6 to generate graphical figures. The difference in grading of inflammatory cells results was performed by student’s t-test in two-tailed test. Values of P< 0.05 were considered statistically significant.
The anti-inflammatory effect of XN with or without PZQ supplements on the liver histopathology of Ov infection and NDMA administration in hamster model. The H&E staining was focused on the localization and aggregation of inflammatory cells surrounding the hepatic bile ducts. In the ON group showed a high aggregation of inflammatory cells on day 60 and decreased on day 90 and returned to increase until day 180 especially in the area where had Ov infection that was the result like the XON group because XN could not eradicate the parasite from bile ducts as shown in Figure 1. Interestingly, in XON group exhibited the numerous of inflammatory cells around bile ducts from day 60 until day 180. However, in ONP and XONP group demonstrated no or only a few inflammatory cells when compared with ON and XON group. From Figure 2 revealed a trend that the inflammatory cells were abundant in ON and XON group when compared with the treated groups. These results indicated that XN in combination with PZQ could inhibit the ongoing inflammatory process precisely.
Figure 1: The histopathology changes of the group infected with Ov and administered with NDMA (ON), presence of xanthohumol (XON), presence of praziquantel (ONP) and presence of xanthohumol and praziquantel (XONP) groups at 60, 90, 120, 180 days respectively that examined by hematoxylin and eosin (H&E) staining in CCA genesis hamsters. An original magnification is ×10 for all figures. BD = Bile duct hyperplasia, IC = Inflammatory cells area, and Ov = Opisthorchis viverrini.
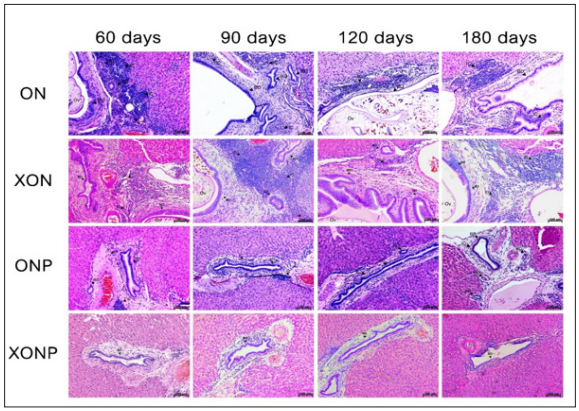
Figure 2: Bar graph comparisons about grading of inflammatory cells in each group of hamster liver; Grade 1: few, Grade 2: moderate, Grade 3: numerous. * P < 0.05 compared to the ON group.

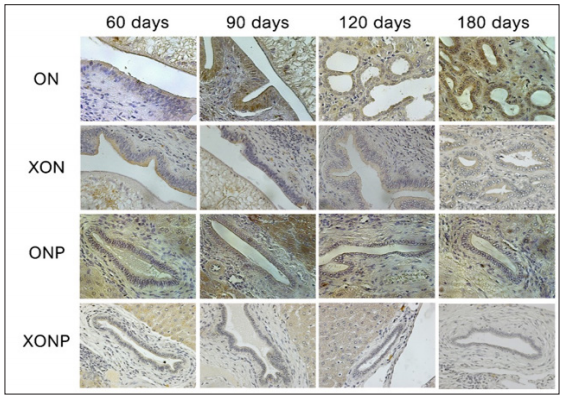
Examples of the immunohistochemical staining results for Twist expression in studied groups as shown in Figure 3. Twist expression was presented in nucleus as well as cytoplasm of hepatocyte, hepatic bile duct and hyperplasia. Our results showed that higher Twist expression was observed along CCA genesis in ON group with time dependent manner. The strongest staining of Twist was found at day 180 in hyperplasia area of the bile duct epithelia in ON group. Interestingly, the reduction of Twist was seen in XON and ONP groups at day 90 when compared with the ON group. Moreover, our results exhibited an effective reduction of Twist expression around bile duct area for the XONP group when compared to all other groups. This was particularly evident in the expression of Twist in XONP group which was reduced from day 90.
Figure 3: Immunohistochemical staining for Twist expression in the group infected with Ov and administered with NDMA (ON), presence of xanthohumol (XON), presence of praziquantel (ONP) and presence of xanthohumol and praziquantel (XONP) groups at 60, 90, 120, 180 days respectively. An original magnification is ×40 for all figures.
At present, the medical approaches for diagnosis, prevention and treatment of CCA are incompetent and establishing novel potential therapeutic ways for CCA should be attempted. Characterization and use of effective natural chemopreventive agents that undertaken by preventing various mechanisms for cancer development have become important issues in cancer researches. Many works informed the anti-carcinogenic features of XN, a natural prenylated chalcone from hop, with a phenomenal broad-spectrum that has distinct inhibitory function in carcinogenesis at the initiation, promotion, and progression stages. Persistent with anti-cancer capability of XN, it strongly regulates the enzymes activity related in detoxification and metabolism of carcinogen. Furthermore, this substance is competent to scavenge oxidative stress as reactive oxygen species and to restrict the production of superoxide anion radical and nitric oxide [25]. In CCA, XN has the inhibitory effects of proliferation and apoptosis in human CCA cell lines through the Notch1/Akt signaling pathway [26].
Additionally in vivo and in vitro study, XN can inhibit the activation of STAT3 in accordance with the Akt-NFκB signaling pathway suppression [26]. Taken together, these data suggested the potential application of XN as a novel, impressively available chemopreventive agent to become a beneficial novel medical approach and strategies for the CCA prevention and treatment. We then investigated the effect of XN on the alteration of histopathology to assess the inflammation in CCA-induced hamster animal model. Our results showed the extensive aggregation of inflammatory cells was found in the group of Ov and NDMA treated hamsters (ON). This related to many works that has been showed the liver fluke can induce chronic inflammatory response, Sripa and coworkers revealed the presence of the Ov antigens was associated with heavy inflammatory cell infiltration, particularly with mononuclear cells [27]. In the treatment of XN alone (XON) found that XN could not reduce the aggregation of inflammatory cells around the hepatic bile ducts but there was an increasing trend of inflammatory cells on day 60, 90, 120, 180 respectively when compared with ON group.
The possible explanation about the type of inflammatory cells that we observed in XON group is M1 macrophages which are normally activated by IFN-γ or lipopolysaccharide (LPS), and produce pro-inflammatory cytokines, phagocytize microbes, and initiate an immune response [28] more than M2 macrophages, tumor-associated macrophages (TAMs) which are actively promote tumor growth [29]. Moreover, our preliminary results showed that M1 macrophages was increased in XON group (data not shown). However, studies should be conducted in depth to prove this hypothesis. In ONP group which was PZQ treated hamsters, the results showed that PZQ could reduce the number of inflammatory cells around the bile duct as well on day 60 and the observation was clear especially on day 90, 120, and 180 respectively. When used XN together with the PZQ (XONP) group it was found to be more effective in reducing inflammatory cells in hamster model.
For the reason that PZQ is effective anthelmintic to eradicate Ov resulting in immune response and inflammatory process were not happened including inflammatory cells. Pinlaor and coworkers indicated that Ov elimination by PZQ treatment reduced bile duct inflammation and oxidative and nitrative DNA damage in acute stage or early stage of opisthorchiasis [30,31]. At present, EMT has been recognized as a potential mechanism related with various types of cancer development. Many works have shown the role of EMT regulators involve in cancer progression and one of these EMT regulator is Twist, it was identified as one of the most key transcription factors that is up-regulated in metastatic tumors [7]. From our immunohistochemical results showed that Twist is overexpressed in ON group at the time dependent manner from day 60 and strongly expressed on day 180. In contrast, the expression of Twist is decreased and absented in the hamster group that treated with XN, PZQ and combination. These results suggested that XN in combination with PZQ could suppress the EMT process by reduced EMT transcription factor expression, Twist efficiently.
According to the study of XN correlated with EMT showed that XN can suppress protein markers of EMT and cell mobility for instance paxillin, MCL2 and S100A4 [32]. The Twist expression results are similar to inflammatory results in experimented hamster group that have mentioned before. Taken together, there was an evidence that Twist was correlated with inflammation in various ways, Twist1 and Twist2 are key regulators of B cell activation in an inflammatory environment such as autoimmune disease [33]. Correspondingly, IFN-induced Twist1 expression was demonstrated to inhibit TNF-α production in macrophages, supporting in down-regulating the inflammatory response [14]. The results of the current study show that XN alone could not reduce inflammatory lesions around bile duct epithelial cells but in combination with PZQ can effectively decrease of inflammatory cell in liver. For EMT, XN and/or PZQ could potentially reduce Twist expression. Further studies are needed to confirm in depth the association between Twist, an EMT regulator and CCA prevention.


