Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Lina Sun*, Kai Cui and Rundong Liang
Received: January 07, 2018; Published: January 10, 2019
*Corresponding author: Lina Sun, College of PE and Sport, Beijing Normal University, Beijing, China
DOI: 10.26717/BJSTR.2019.13.002340
Post-traumatic stress disorder (PTSD) is a serious mental disorder to threaten the life and career of the patients, which highly associated with the neural plasticity at hippocampus. Although previous studies reveal multiple factors acting as the biological bases involving with the development of PTSD, mitochondrial functions could be a new direction for investigating the pathology and therapy of the disease. Recent study suggested improvement of mitochondrial functions is one of the major advantage of physical exercise, which has been widely recognized as the helpful life style in preventing not only psychiatric disorders but also numbers of complex diseases including neurodegeneration, diabetes or even some types of cancer. Thus, mitochondrial functions would be the new target in preventing PTSD or promoting its recovery during treatments. In this mini-review, we summarized the relationship between mitochondrial functions and psychiatric disorder like PTSD and depression. Moreover, based on the benefits of exercise in improving the brain functions via affecting mitochondrial biological status, we proposed that mitochondrial functions are the key to regulate neural functions and to prevent the exacerbation of PTSD.
Keywords: PTSD; Mitochondria; Exercise; Neural plasticity; Hippocampus
Post-traumatic stress disorder (PTSD) is a mental health condition that is triggered by panic memory. It could induce a serious of psychiatric symptoms including avoidance, negative thinking and mood as well as changes in physical and emotional reactions [1,2]. From the angle of pathophysiology, impaired neural plasticity contributes the development of PTSD especially and resulted in the abnormal sensitivity of the patients to certain environmental cues [3,4]. Therefore, how to maintain or reserve neural plasticity and neural symptoms is urgent for solution to develop the effective treatment to PTSD.
Improvement of the neural plasticity especially the adult neurogenesis and synaptic functions at hippocampus is critical to maintain the adaptability of the brain under psychiatric stressors. In adult hippocampus, neural plasticity including neurogenesis and synaptic plasticity are mediated by multiple factors. They serve as the key physiological functions needed for recovered in treating psychiatric disorders like depression, PTSD and bipolar disorder [5-7]. Adult neurogenesis, composed by the self-renewal and fate commitment of neural stem cells (NSCs) as well as the maturation of neural progenitor cells (NPCs), offer the regenerative resource of the neuron population that helps to clear the panic memory [4,8]. Normalized neurogenesis at hippocampus was shown to promote the pattern separation behavior, which enhance the animal to control the emotion in the represented panic scenario [9]. Such capacity is the key for recovery from PTSD. Impairment of neurogenesis results in overgeneralization of fear, which subsequently manifests as inappropriate, uncontrollable expression of fear in neutral and safe environments [10]. Apart from neurogenesis, synaptic plasticity including the synaptogenesis like dendritic spine formation as well as the synaptic functions like long-term potentiation (LTP) and pre-synaptic plasticity also associating with development of depression.
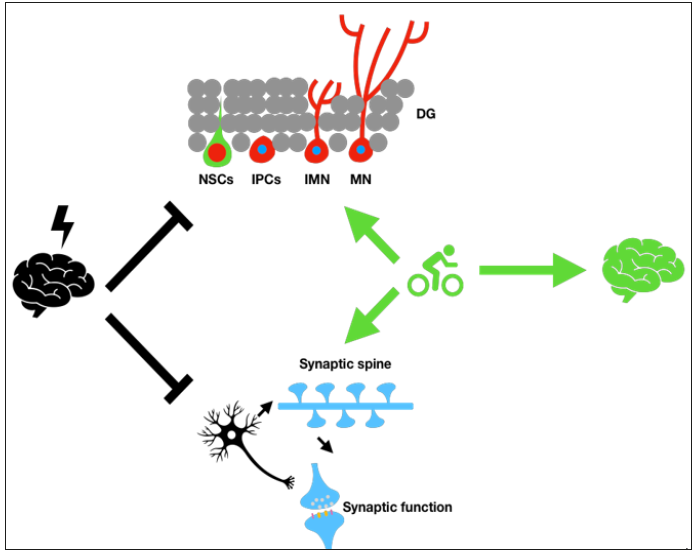
Immobilization-stressed mice presented intensified fear memory and enhanced long-term potentiation (LTP) [11]. Deficiency of brain-derived neurotrophic factor (BDNF) and its intracellular kinase-activating receptor TrkB, have been implicated in the neurobiological mechanisms underlying the clinical manifestations of PTSD [12]. By offering different renewed resource such as aneuronal population as well as synaptic complexity, neural plasticity including neurogenesis and synaptic plasticity support the capacity of the brain to cope with the psychiatric stressors (Figure 1). Conversely, impairment of the neural plasticity is often showing to correlation with the occurrence of psychiatric disorder especially PTSD or major depression [13,14]. Although different efforts were tried to improve the neural plasticity, inefficiency treatment effects or relapse of the disease symptom usually happen due to lack of understanding on the key regulator of the neural plasticity.
Figure 1: Exercise promote the recovery of patients with PTSD by promoting neural plasticity.
Hippocampus is the limbic system in brain with the high structural and functional plasticity. Panic memories due to its strong negative feelings usually form serious psychiatric stressor, which impairs the neural plasticity in hippocampus including neurogenesis and synaptic functions. Neurogenesis mainly including the process of self-renewal of the neural stem cells (NSCs), development of intermediate progenitor cells (IPCs), the maturation of the immature neurons (IMN) as well as the survival of new generated mature neurons (MN). Synaptic plasticity including the density of the post synaptic spines, as well as the functions of single synaptic that containing the functions of pre- and post- synapse. It has been widely reported that exercise could promote those two kinds of neural plasticity and improve the hippocampal functions. Such mechanism explains the effects of exercise in attenuating the main symptoms of PTSD.
The effects of physical exercise, the effective and economic way to prevent and help the recovery the psychiatric symptom associated with PTSD, enlighten the new idea that metabolic functions could be a key target in regulating neural plasticity [15- 17]. Convergent of evidence indicates the role of mitochondria in regulating neural functions. At hippocampus, adult NSCs undergo the switch from glycolysis to oxidative phosphorylation (OXPHOS) following the process of neurogenesis [18]. In mature neurons, mitochondrial metabolism is the main energy source enabling the stimulation of complex electrophysiological process [19,20]. Coincidentally, physical exercise is the effective way to promote mitochondrial functions and perform the therapeutic effects in many chronic diseases, like diabetes, neurodegeneration and psychiatric disorders [21,22]. Additionally, drugs that widely used on clinic was proved could improve the mitochondria functions and thus perform the brain protection.
For example, brain functional recovery drug piracetam was proved could prevent ageing induced neurogenesis decline by promote mitochondrial metabolism [18]. Antioxidants that targeted on controlling the level of reactive oxidative species (ROS) could also reserve the functions of mitochondria [23]. However, it is noteworthy that exercise may elevate the level of ROS, which has been recently declared as the mechanism in antidiabetic effects [24]. Consistent with such point, certain level of ROS serves as the ‘second messenger’ to promote the fate commitment of the NSCs at physiological level [25]. Based on such fact, exercise maybe the effective way to control the level of ROS into physiological reasonable by promoting mitochondrial OXPHOS. For synaptic functions, it was demonstrated that ablation of mitochondrial component uncouple protein 2 (UCP2) induced an anxiety prone, cognitively impaired behavioral phenotype, indicating the key roles of mitochondria functions in regulating synaptic plasticity [26]. Thus, normalization of mitochondrial functions could be the new direction on treatment of PTSD.
It is well-recognized about the benefit of exercise to improve neural functions. However, something that showing beneficial may also lead a harmful result. Likewise, whether overproduction of ROS induced by inappropriate exercise will exacerbate the PTSD associated symptoms remain unknown. With deepen understanding on the relationship between exercise with neural plasticity during the recovery from PTSD, answering the question if certain level of exercise is helpful to improve mitochondrial functions or excessive intensity of exercise can result the adverse effects is worthwhile for explaining. Moreover, how to combine the personality exercise scheme with current clinical medications in different types of PTSD is also need for further investigation. In future research works, focusing on improving mitochondrial functions to promote neuroprotective effects could the breakthrough to treat psychiatric disorders like PTSD and depression.


