Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Roberto Giulianelli*1, Ludovica De Vincentis*1, Barbara Cristina Gentile1, Stefania Monclesi1, Gabriella Mirabile1, Luca Albanesi1, Paola Tariciotti1, Giorgia Tema1, Giorgio Rizzo1, Antonio Nacchia1, Pietro Aloisi1, Giorgio Vincenti1, Riccardo Lombardo2 and Giuseppe Soda1
Received: January 09, 2019; Published: January 17, 2019
*Corresponding author: Roberto Giulianelli, Department of Pathology, Italy
Ludovica De Vincentis, Department of Pathology, Italy
DOI: 10.26717/BJSTR.2019.13.002372
Background: As for other tumors it is likely that depth of invasion is a prognostic factor for disease recurrence and progression in high grade pT1 urothelial bladder cancer. To date nor anatomy based neither dimensional subclassification proved reliable correlation with recurrence and progression, mainly considering the high interobserver variability in pT1 diagnosis, based by the TURB resection technique intrinsic artifact.
Objective: Aim of this study is to assess the feasibility of measuring depth of invasion of urothelial carcinoma in patients undergoing EB-TURB for pT1HG disease.
Design, Setting, and Participants: 27 patients undergoing EB-TURB with Collins knife and with pT1-HG disease were included. A second TURB was performed after 4-6 weeks from the first one. A dedicated pathologist assesses the feasibility of depth of invasion measurement. Outcome Measurements and Statistical Analysis: Evaluate depth of invasion using Kruskal Wallis analysis and Chi-square test.Results and limitations: Overall 32 patients with pT1HG disease were enrolled. EB-resection was adequately performed in 27/32 (85%) of the patients. Overall 40 lesions were identified with a median tumour size of 2 cm (1/4). Median depth of invasion was 1.35 mm (0.48/3.5). Deepness measurement was feasible in 100% of the patients and in 100% of the lesions. As well on re TURB 8/27(29%) patients presented residual disease and in 100% of these patients it was possible to measure depth of invasion with a median value was 1.1 mm (0.43/2.3). Limitations include number of patients.
Conclusion: In specimen obtained from EB-TURB measurement assessment proved to be easy and highly reproducible. Recruitment of patients is still ongoing to evaluate an eventual prognostic value of neoplastic invasion in recurrence and progression. Patient Summary: In this report we want to find out if the thickness of invasion of urothelial carcinoma is related to tumor recurrence and progression measuring depth of invasion.
Keywords: Enbloc-Transurethral Resection of the Bladder; Depth of Invasion; Recurrence
Bladder urothelial carcinoma is the fourth most common cancer in men and the tenth in women [1]. Despite the knowledge of its potential risk, T1 non muscle invasive bladder carcinoma shows a significant variability from patient to patient in term of disease recurrence and progression and consequently in clinical and surgical approach [2]. High grade NMIBC patient’s treatment is the most controversial, especially regarding surgical timing: authors recommend radical cystectomy for all T1, encouraged by the significant risk of progression and the disease specific mortality, thus reserving an overtreatment for non-progressive patients [3], whereas others support the role of adjuvant BCG therapy, with subsequent cystectomy just in case of recurrence [2]. To enlighten which patient should benefit one treatment upon another, increasing interest has been given in study of outcome predictive variable in term of risk of recurrence and progression. EORTC risk table are the best-established models to predict recurrence and progression in NMIBC patients: six clinico-pathological variable including multiplicity, tumor size, T category, tumor grade, prior recurrence rate and presence of concurrent CIS are the most suitable to predict tumor behavior [4]. All these parameters are useful but actually not fully satisfactory in discriminating disease recurrence and progression [5].
Lively debate and active research on this topic moved the focus to lamina propria invasion, investigating the role of muscularis mucosae: stratification by muscularis mucosae invasion is the most studied sub-staging systems and its prognostic value was confirmed by many studies, and even if none have been whole satisfactory, lamina propria invasion is now accepted as a significant predictor of progression in patients with primary pT1 tumour of the bladder, with a 21.9% progression rate for T1 tumours deeply invading the lamina propria [6]. Anyway none of the sub-staging system probed in the past decade has been validated, but despite it is not included in the VII edition of the TNM, it is now strongly recommended by the latest Cancer Staging manual of the American Joint Committee on Cancer to report an attempt to categorize pT1 diseases, whether by measuring the dimension of invasive component quantifying its extent or sub-stage according to relationship to muscularis mucosae/vascular plexus [7,8]. Problems rises also considering the trouble in muscularis mucosae detection in the lamina propria: muscolaris mucosae is an erratic structure, hard to identify on biopsy sample, scattered and discontinuous within the tissue with a documented significant topographical variation in different region of the bladder [9-11]. With current procedure there is no identification of the muscolaris mucosae in biopsy sample in up to 35% of the cases [12].
In this scenario the introduction in routine TURB of novel procedures, such as En-bloc TURB with less artifact and carrying much clear histological information could be helpful in improving our current predictive tool in T1 management: being able to analyze perfectly oriented and intact surgical specimen is one of the possible way to improve any pathological based stratification, allowing a clear identification of high vs. low risk patients and reducing doubt in T1 management. This novel procedure is also safer in term of recurrence: the En-bloc technique provides normal tissue margins around tumor resection border, reducing risk of incomplete resection or at least making easily to identify excision margin, an assessment that was not practicable in conventional piecemeal based TURB [13,14].
The correlation of invasion depth with tumor aggressiveness has been widely experienced (i.e. melanoma Breslow measurement, Depth on invasion DOI in head and neck squamous cell carcinoma, submucosal invasion depth SID in colorectal carcinoma) and presumably the same approach is applicable to high-grade urothelial bladder carcinoma with results similar to that obtained for other malignancies. In this prospective study we aim to improve our outcome prediction tools, testing an old approach to risk stratification on high quality specimen. The main purpose is to find out if the thickness of invasion is related to tumor recurrence and progression. The utility of en-bloc excisional specimen relies on the possibility of tumor orientation, with clear identification of surgical margin on gross examination: this would allow histological correlation between tumors using histologic landmark, since all anatomic structure are easily identifiable on H&E slices from oriented sample.
After an internal review board approval, a consecutive series of patients with bladder neoplasms diagnosed on cystoscopy were prospectively enrolled. All patients signed a dedicated informed consent and the study was conducted in accordance with the principles of the declaration of Helsinki. All patients underwent EB-TURB and only patients with pT1 HG disease are included in our study. A second TURB is performed after 4-6 weeks from the first one. All patients receive detailed clinical history and physical examination. Variables recorded include: sex, age, previous history of bladder cancer, number, location and dimensions of lesions during flexible cystoscopy.
Under regional anaesthesia, patients are placed in a lithotomy position. Routine cystoscopy is performed to confirm the location, number, and volume of the bladder tumour. Physiologic sodium solution is used for irrigation, the en-bloc resection was performed using Collins knife. Intact tumour is flashed out or extracted with a grasper. Thereafter, adequate coagulation is performed at the tumour ground and surrounding mucosa. Catheter is then inserted after verifying no haemorrhage.
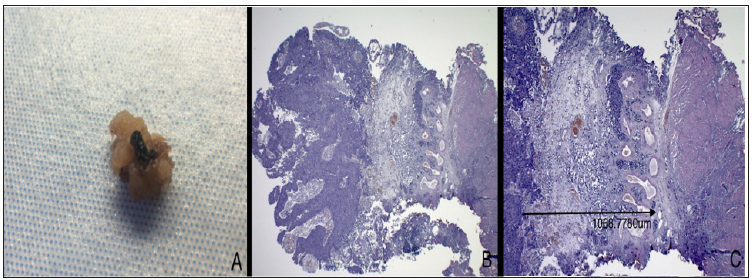
Endoscopic specimen, formalin fixed in 10% buffered formalin solution and unmanipulated for optimum anatomic orientation is sent to pathology lab. Adequate dissection of the gross specimen is essential for accurate measurements: the resected neoplasm is transverse sectioned and whole included in paraffin block and evaluated on ematossiline-eosine section. All slides are digitally acquired using Leica Aperio Scan scope and measured via Aperio Image scope digital slide viewer. Tumor depth is accurately evaluated by examining the deepest infiltration measuring the depth to which tumor cells have invaded submucosa (in mm), starting from the membrane basalis at a right angle, down to the deepest point of invasion (Figure 1).
Figure 1: In A. Gross evaluation of a resected specimen, in this case a polypoid lesion, with easily identifying stalk; the deep surgical line of excision is stained with India Ink for margin status assessment purpose. B. Ematossiline-eosine section at 2,5x magnification, showing a perfectly oriented specimen, with loyal representation and clear low-power identification of lamina propria structures. C. Same slides, 4x magnification, the arrow indicate the measurement of depth of invasion identified as the deepest tumor cell in a perpendicular line to the membrane basalis.
Overall 36 patients with pT1HG disease were enrolled. Out of them EB-resection was adequately performed in 27/36 (75%) of the patients. Median age was 72 (63/78) and overall 23/27 (85%) were males and 5/27 (15%) were females. Data are shown in Table 1. On cystoscopy 16/27 (59%) of the patients presented single tumors while 11/27 (41%) patients presented multifocal lesion. Overall 43 lesions were identified with a median tumor size of 2.cm (1/4) and out of them 36/43 were adequately performed en-bloc. Reasons for not performing the en-bloc technique in the s7 lesions were: 5/7 were electro-fulgurated because to small, 2/7 were neat the ostium.
Depth of invasion measurement was feasible in 100% of the lesions removed with EB-TURB technique. Median depth of invasion was 1.21 mm (0.45/3.24). Overall 6/27 (22%) presented CIS on histological examination. On re-TURB 8/27(29%) patients presented residual disease, 7/8 patients presented pT1HG disease and 1/8 patients presented pTaLG disease on re-TURB. In the 8 patients a total of 10 lesions were resected en-bloc. In 100% of these patients it was possible to measure depth of invasion and the median value was 0.61 mm (0.21/2.2).
According to actual WHO/ISUP 2016 classification, the group of non-invasive carcinoma comprises tumor with similar biological property as invasive urothelial carcinoma, underscoring the high risk of progression of these pathological entity, ranging from 21 to 50% [8]. Considering the high variability in clinical outcome within this group of tumor and the lack of well-established risk assessment criteria for recurrence and progression, treatment of pT1 high-grade bladder carcinoma is still challenging, making urgent the needing of individuating outcome predictive elements leading to standardization of management. Along with classical clinical parameter (multiplicity, tumor size, T category, tumor grade, prior recurrence rate and presence of concurrent CIS) histological substaging system has been widely explored and recently depth of invasion has been advocated as the most important prognostic factor in term of disease progression and cancer specific survival [15]. Over the past 20 year subclassification of pT1 stages according to muscularis mucosae invasion seemed to be the most reasonable method for stratification purpose; recently also dimensional approaches proved to be a powerful predictor of tumoral behavior, but to date no definite correlation between neoplastic invasion and time to recurrence and progression has been clearly identified.
In 1990’s Younes was the first to introduce the needing of sub-staging system with prognostic influence in T1 bladder cancer individuating three categories: tumoral invasion located above (a), into (b) and beyond (c) the muscularis mucosae [6]. His group found a significant role of lamina propria invasion in outcome prediction of T1 tumors with a 75% 5 year survival for T1a and T1b and an 11% for T1c opening the way to many other different systems in order to get the best fitting subdivision of this heterogeneous group of tumor. Other anatomy-based systems sub-classify NMIBC according to presence of cancer invasion near muscularis mucosae (pT1b) or absence (pT1a) [16] or more simply in above or into (pT1a) and beyond (pT1b) [17]. Lawless et al. focusing on papillary exophytic subtype with its peculiar stalk architecture, proposes to sub-stage by separating invasion of the lamina propria into the stalk from invasion of the base, either focal or extensive (basing on a 1 mm threshold), with significant results in term of progression [18]. Along these lines other used a sizebased approach: Chang measurement focus on maximum extent of invasion [19], while other method recognizes microinvasive (pT1-m) versus extensive (pT1-e) invasive bladder tumor in 1 highpower field with a 0,5 mm threshold [20]. Recently the 1 mm cut-off in 20x HPF multidirectional measurement adopted in the ROL (Rete Oncologica Lombarda) system [21] proved to be feasible and highly reproducible.
However, the lack of uniformity between these methods make data comparison difficult and to date no reliable correlation in term of disease recurrence and progression has been found. Must be considered that pathological staging of urothelial bladder carcinoma on specimens obtained with traditional TURB is often inaccurate due to fragmented tumor samples, with poor anatomic orientation, severe thermal artifact and crush effects related to the surgical procedure. These technique intrinsic artifacts are most likely the main reason why all measurement based sub staging system failed [22,23] and lead also to high inter-observer variability with subsequent risk of understaging and misleading therapeutic decisions. Since its introduction by Edwin Beer [24] endoscopic management of bladder tumor remains the Gold standard, and to date, TURB techniques allow not only diagnosis but, in many cases, definitive treatment by mean of total removal of bladder cancer. According to Herr quality assessment of a good TURB must evaluate the complete resection, presence of deep muscle in the sample and the rate of recurrence [25]. Despite its long standing application, even in experienced specialist, TURB resection is sometimes inadequate: consider the case of multiple tumor, tumors with large bulk, or patients features such as habitus or aesthetic risk, or even procedure inherent complication such as bleeding or bladder perforation [26,27].
In surgical practice despite the great variation in techniques and quality of TURB no consensus has been found in adequacy of Turb specimen sampling [28]. Both CUA (Canadian Urological Association) and NICE (National Institute for Health and Care Excellence) guidelines emphasize the requirement of obtaining muscle within the specimen. NICE also recommends repeating the procedure within 6 weeks if histologically none is identified [29]. Although the complete one time removal of all bladder cancer by TURB is the goal, this is not always easy to reach. A second TURB is recommended for the following cases: incomplete initial TURB, no muscle in the specimen after initial resection (with exception of Ta low grade tumors), in case of T1 tumor and all high grade tumors [30]. Increasing evidence supports EB-TURB improvement of specimen quality; the presence of muscular propria in neoplastic resection is crucial for correct diagnosis and in one study detrusor muscle was clearly identified in 94.4% initial specimen from En-Bloc TURBT, whereas only in 60% from conventional TURB biopsy [14]. In a pathological point of view En Bloc TURB can both improve the surgical management, reducing the needing of a second look TURB, and facilitate the sample evaluation, especially for what concern depth of tumor invasion and margins status, allowing accurate histological based risk stratification. In the present study we proved the feasibility of neoplastic depth measurement when performing EB-TURB. In this pilot study all the resected lesions it was passible of depth of invasion evaluation. Moreover, the measurement was simple and straightforward. However, it is important to underline that in 25% of the patients it was not possible to perform an EBTURB and were excluded from the study because of large tumour (>5cm) and fragmentation of the tissue when removed from the bladder. The present study is the only available evidence to our knowledge describing the measurement in pT1 high grade bladder cancer of invasion deepness on EB-TURB specimens.
Despite the paucity of high-level evidence regarding correlation of neoplastic invasion with recurrence and progression in pT1- NMIBC, histological sub-staging is still attractive and the latest AJCC Cancer staging manual strongly recommend reporting further information in pT1 diagnosis record, encouraging anatomybased subclassification and tumoral invasion measurements. We are deeply confident that the introduction in common surgical practice of much representative tumor sample, obtained with tissue respectful techniques such as en-bloc TURB, could improve our outcome prediction tools: the possibility to analyze perfectly oriented specimen, with easily recognizable lamina propria structure and no doubt in invasion assessment trough reproducible measurement method, is the only way to compare tumor, and validate or disprove the role of neoplastic invasion thickness in mm.
In line with other epithelial malignancies, staging according to depth of invasion hold the promise to improve our understanding in this heterogeneous group of neoplasm. Target of this prospective study is to get over problems affecting the TURB procedure in order to verify if tumoral thickness is actually an outcome predictive variable, and if so, to set a significant cut-off. The precise topographical localization of neoplastic cell and the clear identification of tumoral relationship with submucosal structures would surely reduce interobserver variability in pT1 diagnosis, with no doubt in invasion assessment and measurement, allowing reliable stratification of recurrence and progression and thus providing basis for rational therapeutic approach to high-grade non muscle invasive carcinoma behavior. Comparison of measurements will eventually reveal statistical correlation between the depth of invasion measurement and other validated clinical parameters or assess a role as independent prognostic factor for the histological parameter.
To appreciate the potential benefit of this approach it would be necessary to enroll a large number of patients. At the time of submission due to the limited follow-up no correlation is allowed yet, but histological information obtained from en-bloc TURB are so thick that we are truly confident will confirm our expectation.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors.


