Abstract
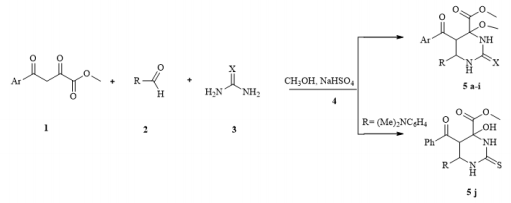
Methyl 5-aroyl-6-aryl-4-methoxy-2-oxo(thioxo)hexahydropyrimidine-4-carboxylates were synthesized by four-component Biginelli’s reaction of methyl aroylpyruvate, aromatic aldehyde, urea (thiourea) and methanol in presence the sodium hydrogen sulfate . Products were obtained in moderate to high yields.
Keywords: Biginelli’s Reaction; Methyl Aroylpyruvate; Aromatic Aldehyde; Urea; Thiourea; Sodium Hydrogen Sulfate; Methanol; Methyl 5-Aroyl-6-Aryl-4-Methoxy(Hydroxy)-2-Oxo(Thioxo)Hexahydropyrimidine-4-Carboxylates
Introduction
In 1893 year, three-component acid-catalyzed condensation of ethyl acetoacetate, benzaldehyde, and urea for synthesis of 3,4-dihydropyrimidin-2(1H) ones (DHPMs) was reported by an Italian chemist Pietro Biginelli [1]. The great interest of chemists in Biginelli condensation provided access to many multi functionalized dihydropyrimidines due to the expansion of synthetic potentials of the well-known Biginelli reaction [2]. It is known that 5-functionally-substituted dihydropyrimidin-2(1H)ones (thiones) (5-carbonyl-, 5-alkoxycarbonyl-, 5-carbamoyl-, 5-carbonitryl-, 5-nitro-) have a wide range of biological activity (Figure 1). Among them was found compounds with antitumor [3], anti-inflammatory [4], anti-tuberculosis [5], antimycobacterial [6], antioxidant [7], fungicidal [8], antihypertensive [9] activities. Some of them are used as calcium channel blockers [10]. The biological screening showed prospects of using of known N(3)-substituted 5-alkoxycarbonyldihydropyrimidin-2(1H)ones(thiones) as vasodilatators (3-alkoxycarbonyl-),[11] cardiovascular (3-alkyl-, 3-acyl-) [12] antimalarial [13] (3-carbamoyl-), anti-inflammatory (3-methyl-, phenazyl-)[14] agents and of antagonists of adrenergic receptors [15]. Among the N(1)-substituted 5-carbonyldihydropyrimidin-2(1H)ones(thiones) show carcinostatics (1-alkyl-) [16].
The chemical modification of the pyrimidine ring, by replacing the original components of the classic Biginelli’s reaction, can lead to strengthening or changing the pharmacological properties, reduce toxicity and given the practical significance of pyrimidine derivatives, chemists’ interest in finding a new ways to modify of the pyrimidine cycle to produce new pyrimidine derivatives with potential biological activity is relevant. We previously studied the possibility of using methyl aroylpyruvates in reaction Biginelli with aromatic aldehydes, and urea in the absence of a solvent and a catalyst and received of methyl 6-aryl-5-aroyl-2-oxo-1,2,3,6-tetrahydropyrimidine-4-carboxylates as the only products [17]. Data on the synthesis of DHPMs with two substituents at the 4-position of the pyrimidine ring are scarce. Previously, the authors reported the formation of 4-hydroxytetrahydropyrimidinones in the reaction Biginelli if the structure of the dicarbonyl compound contain strong electron-acceptor substituents [18]. In preceding publication, we described the formation of 5-hydroxy-4,5,6,7-tetrahydrotetrazolo[1,5-а]pyrimidines in the reaction of methyl acetylpyruvate, aromatic aldehyde, and 5-aminotetrazole monohydrate [19]. We recently discovered and reported a simple way of obtaining non-dehydrated reaction products of Biginelli’s reaction with simultaneous methylation of the tertiary hydroxyl group by reaction of methyl benzoylpyruvate with an aromatic aldehyde and urea in the presence of nontoxic, cheap sodium hydrogen sulfate as catalyst with formation three examples of methyl 5-benzoyl-6-aryl-4-methoxy-2-oxohexahydropyrimidine-4-carboxylates. The reaction was carried out by heating in methanol [20].
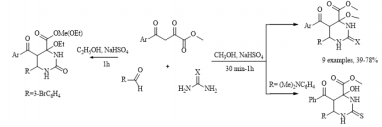
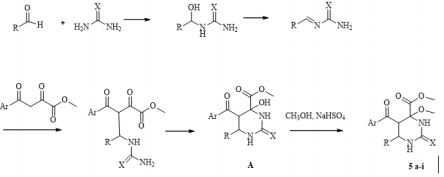
Continuing research in this area, in this paper, we extended the range of aromatic aldehydes in the reaction with methyl aroylpyruvates and urea and investigated the possibility of replacing urea with thiourea in this reaction. It was found that the reaction similarly stops at the stage of formation of an undehydrated product with methylation of a tertiary hydroxyl group and as a result, we isolated new noncharacteristic products for Biginelli’s reaction, in good yields (Scheme 1). 5: R= 4-HO C6H4 (a), 3-MeOC6H4 (b), 4-MeOC6H4 (c, g), 2,4-(MeO)2C6H3 (d), 4-Cl C6H4 (e, h), 4-F C6H4 (f, i), (CH3)2N C6H4 (j); Ar = Ph (a-d, g-j), 4-CH3 C6H4 (e, f), X = O (af), S (g-j). Despite the large number of publications on the study of the Biginelli’s reaction, its mechanism is still under debate. Several mechanisms for the acid-catalyzed reaction have been reported. In this case a suggested mechanism is depicted in Scheme 2 and at the stage of the formation of 6-aryl-5-aroyl-4-hydroxy-2-oxohexahydropyrimidine-4-carboxylate a methylation occurs of the hemiaminal hydroxy group catalyzed by acidic by the nature sodium hydrogen sulfate. The electron-acceptor methoxycarbonyl and benzoyl groups in the positions 4 and 5 of the heterocycle stabilize structure А [21]. The reaction of obtaining the compound 5j stops at the stage of formation of structure А due to the basic properties of dimethylamino group of aromatic aldehyde, which possibly blocks the action of the catalyst.
Scheme 1: Synthesis of methyl 5-aroyl-6-aryl-4-methoxy-2-oxo(thioxo)hexahydropyrimidine-4-carboxylates (5a-i).
Scheme 2: Proposed mechanism for the formation of methyl 5-aroyl-6-aryl-4-methoxy-2-oxo(thioxo)hexahydropyrimidine-4- carboxylates.
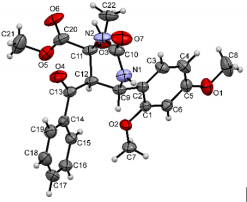
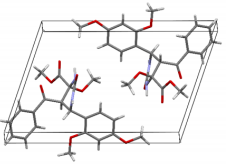
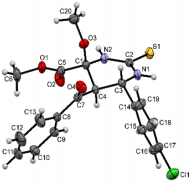
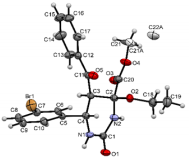
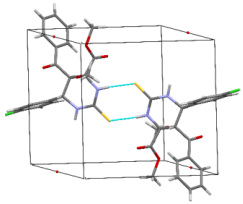
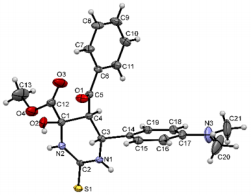
The structures were confirmed by 1 H NMR, 13C NMR and IR spectroscopy, as well as elemental analysis. Additionally, the structures of methyl 5-benzoyl-4-methoxy-6-(2,4-dimethoxyphenyl)-2-oxohexahydropyrimidine-4-carboxylate (5d), methyl 5-benzoyl-6-(4-chlorophenyl)-4-methoxy-2-thioxohexahydropyrimidine-4-carboxylate (5h), methyl 5-benzoyl-6-(4-(dimethylamino)phenyl)-4-hydroxy-2-thioxohexahydropyrimidine-4-carboxylate (5j) were confirmed by single crystal X-ray analysis (Figures 2- 4). Substitution of methanol for ethanol in this reaction led to ethylation of the tertiary hydroxy group and partial transesterification of methoxycarbonyl group (Scheme 3). The structure of methyl(ethyl) 5-benzoyl-6-(3-bromophenyl)-4-ethoxy-2-oxohexahydropyrimidine-4-carboxylate were confirmed by 1H NMR and single crystal X-ray analysis (Figure 5). CCDC 1833000 (for 5d), CCDC 1832999 (for 5h), CCDC 1832998 (for 5j) and CCDC 1832997 (for 6a), contain the supplementary crystallographic data for this paper. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk. In summary, we have identified new possibilities for the Biginelli reaction, which allow us to introduce the methoxy group into the 4-position of the heterocycle by four-component Biginelli’s reaction, which allows us to significantly expand the range of use of Biginelli reaction in the synthesis of biologically active compounds. For the first time shown the influence of the catalyst, the nature of the substituent in aromatic aldehyde and solvent on the structure of the obtained DHPMs. The obtained compounds can be used for the synthesis of condensed heterocyclic compounds (Figure 6).
Figure 2: Molecular structure of compound 5d showing 50% probability amplitude displacement ellipsoids (CCDC 1833000).
Figure 4: Molecular structure of compound 5h showing 50% probability amplitude displacement ellipsoids (CCDC 1832999).
Figure 5: Molecular structure of compound 6a showing 50% probability amplitude displacement ellipsoids (CCDC 1832997). The minor disorder component is shown with white carbons.
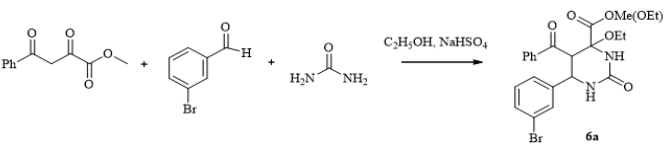
Scheme 3: Synthesis of methyl(ethyl) 5-benzoyl-6-(3-bromophenyl)-4-ethoxy-2-oxohexahydropyrimidine-4-carboxylate (6a).
Experimental Section
All reagents and solvent were commercially available with analytical grade and used as received. Melting points were taken on a M-565. Infrared spectra (IR) were obtained on a Specord M-80 spectrometer in KBr. The 1H NMR spectra were recorded on a Bruker 300 instruments using DMSO-d6 as solvent. The unit cell parameters and the X-ray diffraction intensities were measured on a Xcalibur Ruby diffractometer. The unit cell parameters and the X-ray diffraction intensities were measured on a Xcalibur Ruby diffractometer (Figure 7). The empirical absorption correction was introduced by multi-scan method using SCALE3 ABSPACK algorithm [21]. The structures were solved by charge flipping algorithm using the Superflip program [22] (5h, 5j) or olex [2]. solve program [23] (6a) or by direct method using the SHELXS-97 program [24] (5d) and refined by the full-matrix least-squares method in the anisotropic approximation for all non-hydrogen atoms using the SHELX-2014 [25] and OLEX2[26] program packages. Hydrogen atoms bound to carbon were located from the Fourier synthesis of the electron density and refined using a riding model. The hydrogen atoms of NH and OH groups were refined freely with isotropic displacement parameters. The H atoms of water molecule (5h) could not be located in the difference Fourier map or placed geometrically, and therefore were omitted from the refinement [27-32].
Figure 7: Molecular structure of compound 5j showing 50% probability amplitude displacement ellipsoids (CCDC 1832998).
Representative Procedure General Procedure for the Synthesis of Methyl 5-Aroyl-6-Aryl-4-Methoxy-2-Oxo(Thioxo) Hexahydropyrimidine-4-Carboxylates(5a-I): A mixture of methyl aroylpyruvate (1) (0.01 mol), aromatic aldehyde (2) (0.01 mol) and urea (thiourea) (3) (0.015 mol) and MeOH (10 mL) (4) in presence the NaHSO4 (0.008 mol) in was heated at for 30 min- 1 h. After reaction completion, the resulting precipitate was collected by filtration and washed with water, the crude product was recrystallized from MeOH (Figure 8).
The Procedure for the Synthesis of Methyl(ethyl) 5-Benzoyl-6-(3-Bromophenyl)-4-Ethoxy-2-Oxohexahydropyrimidine-4-Carboxylate (6a): A mixture of methyl benzoylpyruvate (1) (0.01 mol), 3-bromobenzaldehyde (2) (0.01 mol) and urea (3) (0.015 mol) and EtOH (10 mL) (4) in presence the NaHSO4 (0.008 mol) in was heated at for 1 h. After reaction completion, the resulting precipitate was collected by filtration and washed with water, the crude product was recrystallized from EtOH.
Methyl5-Benzoyl-6-(4-Hydroxyphenyl)-4-Methoxy-2-Oxohexahydropyrimidine-4-Carboxylate (5a): Yield: 2.46 g (64%), colorless crystalline solid, mp 252-256°C; 1 H NMR (300 MHz, DMSO-d6) δ, ppm (J, Hz): 3.09 (3 H, s, COOCH3 ); 3.19 (3 H, s, CH3 O); 4.25 (1 H, d, J = 11 Hz, H-5); 4.91 (1 H, d, J = 11 Hz, H-6); 6.59 (2 H, d, J = 8.4 Hz, ArH); 6.72 (1 H, s, 1-H); 7.19 (2 H, d, J = 8.4 Hz, ArH); 7.39 (2 H, t, J = 8.4 Hz, ArH); 7.52 (1H, t, J = 8.4 Hz, ArH); 7.59 (2H, d, J = 8.4 Hz, ArH); 7.76 (1H, s, 3-H); 9.14 (1H, s, OH) ppm; Found, %: С, 62.61; Н, 5.33; N, 7.16. Anal. Calcd for С20H20N2O6: С, 62.49; Н, 5.24; N, 7.29%.
Methyl-5-Benzoyl-6-(3-Methoxyphenyl)-4-Methoxy-2-Oxohexahydropyrimidine-4-Carboxylate (5b): Yield: 2.70 g (68%), colorless crystalline solid, mp 162-164°C. IR (KBr) ν (cm-1) 1664, 1724, 3168, 3354. 1 H NMR (300 MHz, DMSO-d6) δ, ppm (J, Hz): 3.09 (3 H, s, COOCH3 ); 3.18 (3 H, s, CH3O); 3.70 (3 H, s, CH3O); 4.39 (1 H, d, J = 11 Hz, H-5); 5.03 (1 H, d, J = 11 Hz, H-6); 6.74 (1 H, d, 1-H); 6.98 (1 H, s, ArH); 7.00 (2 H, d, J = 7.8 Hz, ArH); 7.15 (1 H, t, J = 7.5 Hz, ArH); 7.42 (2 H, t, J = 7.8 Hz, ArH); 7.55 (1 H, t, J = 7.2 Hz, ArH); 7.67 (2 H, d, J = 7.2 Hz, ArH); 7.95 (1 H, s, 3-H) ppm. Found, %: С, 63.45; Н, 5.66; N, 7.14. Anal. Calcd for С21H22N2O6: С, 63.31; Н, 5.57; N, 7.03%.
Methyl5-Benzoyl-4-Methoxy-6-(4-Methoxyphenyl)-2-Oxohexahydropyrimidine-4-Carboxylate (5c): Yield: 2.35 g (59%), colorless crystalline solid, mp 224-226°C. 1 H NMR (300 MHz, DMSO-d6) δ, ppm (J, Hz): 3.10 (3 H, s, COOCH3 ); 3.19 (3 H, s, CH3 O); 3.65 (3 H, s, CH3 O); 4.28 (1 H, d, J = 11 Hz, H-5), 4.98 (1 H, d, J = 11 Hz, H-6); 6.70 (1 H, s, 1-H); 6.78 (2 H, d, J = 8.7 Hz, ArH); 7.32 (2 H, d, J = 8.7 Hz, ArH); 7.39 (2 H, t, J = 7.2 Hz, ArH); 7.52 (1 H, t, J = 7.2 Hz, ArH); 7.62 (2 H, d, J = 7.2 Hz, ArH); 7.71 (1 H, s, 3-H) ppm. 13С NMR (75 MHz, DMSO-d6) δ, ppm: 50.59; 51.65; 51.73; 53.05; 54.88; 85.54; 113.47; 127.46; 128.39; 129.18, 131.67; 132.85; 137.56; 153.19; 158.72; 167.73; 194.20. Found, %: С, 63.43; Н, 5.48; N, 7.16. Anal. Calcd for С21H22N2O6 : С, 63.31; Н, 5.57; N, 7.03%.
Methyl5-Benzoyl-4-Methoxy-6-(2,4-Dimethoxyphenyl)-2-Oxohexahydropyrimidine-4-Carboxylate (5d): Yield: 2.65 g (62%), colorless crystalline solid, mp 209-211°C. 1 H NMR (300 MHz, DMSO-d6) δ, ppm (J, Hz): 2.79 (3 H, s, COOCH3); 3.50 (3 H, s, CH3O); 3.75 (3 H, s, CH3 O); 3.87 (3 H, s, CH3O); 4.48 (1 H, br s, H-5); 4.76 (1 H, br s, H-6); 6.51 (1 H, d, J = 8.7 Hz, ArH); 6.56 (1 H, s, ArH); 6.69 (1 H, s, 1-H); 6.93 (1 H, s, 3-H); 7.06 (1 H, d, J = 8.4 Hz, ArH); 7.61 (2 H, t, J = 6.9 Hz, ArH); 7.70 (1 H, t, J = 7.2 Hz, ArH); 8.04 (2 H, d, J = 7.2 Hz, ArH). Found, %: С, 61.80; Н, 5.40; N, 7.05. Anal. Calcd for С22H24N2O7 : С, 61.68; Н, 5.65; N, 6.54%.
Crystal Data of 5d. С22H24N2O7 , M = 428.43, triclinic, a = 7.3891(9) Å, b = 10.4223(12) Å, c = 15.1407(17) Å, α = 69.918(10) °, β = 81.948(10) °, γ = 69.871(11) °, V = 1027.9(2) Å3, T = 295(2), space group P–1, Z = 2, μ(Mo Kα) = 0.104 mm-1. The final refinement parameters: R1 = 0.0513, wR2 = 0.1278 [for observed 3733 reflections with I > 2σ(I)]; R1 = 0.0670, wR2 = 0.1451 (for all independent 4869 reflections, Rint = 0.0358), S = 1.025. Largest diff. peak and hole 0.230 and –0.308 ēÅ-3.
Methyl 6-(4-Chlorophenyl)-4-Methoxy-5-(4-Methylbenzoyl)-2-Oxohexahydropyrimidine-4-Carboxylate (5e): Yield: 2.96 g (71%), colorless crystalline solid, mp 218-220°C. 1 H NMR (300 MHz, DMSO-d6) δ, ppm (J, Hz): 2.30 (3 H, s, CH3 ); 3.14 (3 H, s, COOCH3 );3.19 (3 H, s, CH3 O); 4.28 (1 H, d, J = 11 Hz, H-5); 5.00 (1 H, d, J = 11 Hz, H-6); 6.83 (1 H, s, 1-H); 7.21 (2 H, d, J = 8.4 Hz, ArH); 7.26 (2 H, d, J = 8.7 Hz, ArH); 7.42 (2 H, d, J = 8.7 Hz, ArH); 7.54 (2 H, d, J = 8.4 Hz, ArH); 7.73 (1 H, s, 3-H) ppm. 13С NMR (75 MHz, DMSO-d6) δ, ppm: 20.88; 50.64; 51.32; 51.86; 53.45; 85.60; 127.65; 127.97; 128.37; 128.85; 129.83; 132.11; 138.85; 142.94; 153.18; 167.64; 193.48. Found, %: С, 60.38; Н, 5.17; N, 6.60.Anal.Calcd for С21H21ClN2O5 : С, 60.51; Н, 5.08; N, 6.72%.
Methyl 6-(4-Fluorophenyl)-4-Methoxy-5-(4-Methylbenzoyl)-2-Oxohexahydropyrimidine-4-Carboxylate (5f): Yield: 2.96 g (74%), colorless crystalline solid, mp 226-228°C. 1H NMR (300 MHz, DMSO-d6) δ, ppm (J, Hz): 2.29 (s, 3 H, CH3 ); 3.14 (3 H, s, COOCH3 ); 3.19 (3 H, s, CH3 O); 4.28 (1 H, d, J = 11 Hz, H-5); 5.00 (1 H, d, J = 11 Hz, H-6); 6.82 (1 H, s, 1-H);7.00 (2 H, t, J = 8.7 Hz, ArH); 7.20 (2 H, d, J = 8.1 Hz, ArH); 7.40-7.45 (2H, m, ArH); 7.54 (2H, d, J = 8.1 Hz, ArH); 7.74 (s,1H, 3-H) ppm. 13С NMR (75 MHz, DMSO-d6) δ, ppm: 20.74; 50.52; 51.45;51.73; 53.03; 85.54; 114.47; 114.75; 127.49; 128.82; 129.91; 130.02; 135.05; 135.87; 135.97;143.29; 153.09; 159.81; 163.04; 167.59; 193.42. Found, %: С, 62.87; Н, 5.37; N, 7.11. Anal. Calcd for С21H21FN2O5 : С, 62.99; Н, 5.29; N, 7.00% (Figure 9).
Methyl 5-Benzoyl-4-Methoxy-6-(4-Methoxyphenyl)-2-Thioxohexahydropyrimidine-4-Carboxylate (5g): Yield: 2.81 g (68%), colorless crystalline solid, mp 206-208°C. 1 H NMR (300 MHz, DMSO-d6) δ, ppm (J, Hz): 3.14 (3 H, s, COOCH3 ); 3.22 (3 H, s, CH3 O); 3.66 (3 H, s, CH3 O); 4.38 (1 H, d, J = 11 Hz, H-5); 4.93 (1 H, d, J = 11 Hz, H-6); 6.81 (2 H, d, J = 8.7 Hz, ArH); 7.33 (2 H, d, J = 8.7 Hz, ArH); 7.39 (2 H, t, J = 7.5 Hz, ArH); 7.54 (1 H, t, J = 7.5 Hz, ArH); 7.63 (2 H, d, J = 7.2 Hz, ArH); 8.48 (1 H, s, 1-H); 9.20 (1 H, s, 3-H) ppm. 13С NMR (75 MHz, DMSO-d6) δ, ppm: 49.88; 51.38; 54.01; 54.91; 51.92; 84.11; 113.57; 127.63; 128.3; 129.42; 130.16; 133.12; 137.21; 158.93; 166.84; 175.86; 193.77. Found, %: С, 60.98; Н, 5.25; N, 6.65. Anal. Calcd for С21H22N2O5 S: С, 60.85; Н, 5.35; N, 6.76%.
Methyl 5-Benzoyl-6-(4-Chlorophenyl)-4-Methoxy-2-Thioxohexahydropyrimidine-4-Carboxylate (5h): Yield: 3.14 g (75%), colorless crystalline solid, mp 238-240°C. IR (KBr) ν (cm-1): 1684, 1731, 3169, 3379. 1 H NMR (300 MHz, DMSO-d6) δ, ppm (J, Hz): 3.14 (3 H, s, COOCH3 ); 3.23 (3 H, s, CH3 O); 4.42 (1 H, d, J = 11 Hz, H-5); 4.99 (1 H, d, J = 11 Hz, H-6); 7.30 (2 H, d, J = 8.4 Hz, ArH); 7.37 (2 H, d, J = 8.4 Hz, ArH); 7.43 (2 H, t, J = 8.4 Hz, ArH); 7.55 (1 H, t, J = 7.2 Hz, ArH); 7.66 (2 H, d, J = 7.2 Hz, ArH); 8.75 (1 H, s, 1-H); 9.26 (1 H, s, 3-H) ppm. 13С NMR (75 MHz, DMSO-d6) δ, ppm: 50.40; 50.80; 52.04; 53.96; 80.48; 127.74; 128.06; 128.35; 130.16; 133.12; 137.27; 137.44; 168.59; 175.82; 195.25. Found, %: С, 57.23; Н, 4.48; N, 6.56. Anal. Calcd for С20H19ClN2O4S: С, 57.35; Н, 4.57; N, 6.69%. Crystal Data of 5h. 2С20H19ClN2O4 S∙O, M = 853.76, triclinic, a = 8.0431(14) Å, b = 10.3361(18) Å, c = 12.506(2) Å, α = 88.241(14) °, β = 86.090(14) °, γ = 79.319(15) °, V = 1019.2(3) Å3, T = 295(2), space group P–1, Z = 1, μ(Mo Kα) = 0.321 mm-1. The final refinement parameters: R1 = 0.0610, wR2 = 0.1520 [for observed 3057 reflections with I > 2σ(I)]; R1 = 0.0990, wR2 = 0.1864 (for all independent 4713 reflections, Rint = 0.0441), S = 1.042. Largest diff. peak and hole 0.446 and –0.368 ēÅ-3.
Methyl 5-Benzoyl-6-(4-Fluorophenyl)-4-Methoxy-2-Thioxohexahydropyrimidine-4-Carboxylate (5i): Yield: 3.13 g (78%), colorless crystalline solid, mp 288-290°C. 1 H NMR (300 MHz, DMSO-d6) δ, ppm (J, Hz): 3.14 (s, 3 H, COOCH3 ); 3.23 (s, 3 H, CH3 O); 4.42 (1 H, d, J = 11 Hz, H-5); 4.99 (1 H, d, J = 11 Hz, H-6); 7.04 (2 H, t, J = 8.7 Hz, ArH); 7.37-7.48 (4 H, m, ArH); 7.54 (1 H, t, J = 7.2 Hz, ArH); 7.64 (2 H, d, J = 7.2 Hz, ArH); 9.24 (1 H, s, 3-H); 8.71 (1 H, s, 1-H) ppm. 13С NMR (75 MHz, DMSO-d6) δ, ppm: 50.03; 51.39; 51.90; 54.01; 84.12; 114.72; 115.00; 127.61; 128.41; 130.30; 130.41; 133.13; 134.40; 134.44; 137.22; 160.06; 163.29; 166.74; 176.14; 193.76. Found, %: С, 59.84; Н, 4.84; N, 6.83.Anal.Calcd for С20H19FN2 O4 S: С, 59.69; Н, 4.76; N, 6.96%.
Methyl5-Benzoyl-6-(4-(Dimethylamino)phenyl)-4-Hydroxy-2-ioxohexahydropyrimidine-4-Carboxylate (5j): Yield: 1.61 g (39%), colorless crystalline solid, mp 140-142°C. 1 H NMR (300 MHz, DMSO-d6) δ, ppm (J, Hz): 2.81 (6 H, s, N(CH3 )2 ); 3.20 (3 H, s, COOCH3 ); 4.35 (1 H, d, J = 11 Hz, H-5); 4.89 (1 H, d, J = 11 Hz, H-6); 6.61 (2 H, d, J = 8.7 Hz, ArH); 6.88 (1 H, s, OH); 7.18 (2 H, d, J = 8.7 Hz, ArH); 7.38 (2 H, t, J = 7.5 Hz, ArH); 7.51 (1 H, t, J = 7.5 Hz, ArH); 7.64 (2 H, d, J = 7.5 Hz, ArH); 8.22 (1 H, s, 1-H); 8.61 (1 H, s, 3-H) ppm. Found: С, 61.12; Н, 5.70; N, 10.29%. Anal. Calcd for С21H23N3 O4 S: С, 61.00; Н, 5.61; N, 10.16%. Crystal Data of 5j. С21H23N3 O4 S, M = 413,48, triclinic, a = 5.7157(15) Å, b = 12.854(3) Å, c = 15.156(2) Å, α = 105.660(15) °, β = 96.970(17) °, γ = 99.642(19) °, V = 1040.6(4) Å3, T = 295(2), space group P–1, Z = 2, μ(Mo Kα) = 0.188 mm-1. The final refinement parameters: R1 = 0.0573, wR2 = 0.1503 [for observed 4382 reflections with I > 2σ(I)]; R1 = 0.0863, wR2 = 0.1597 (for all independent 6732 reflections), S = 0.969. Largest diff. peak and hole 0.388 and –0.302 ēÅ-3. The crystal is a nonmerohedral twin, the refined ratio of the twin components is 0.5635(14):0.4365(14).
Methyl (ethyl) 5-benzoyl-6-(3-bromophenyl)-4-ethoxy2-oxohexahydropyrimidine-4-Carboxylate (6a): Yield: 2.91 g (63%), colorless crystalline solid, mp 198-200°C. 1 H NMR (300 MHz, DMSO-d6) δ, ppm (J, Hz): 0.86 (3 H, t, COOC2 H5 ); 1.09 (3 H, t, OC2 H5 ); 3.32 (2 H, q, OC2H5); 3.53 (2 H, q, COOC2 H5 ); 3.04 (3 H, s, COOCH3 ); 4.37 (1 H, d, J = 11 Hz, H-5); 5.04 (1 H, d, J = 11 Hz, H-6); 7.10 (1 H, s, 1-H); 7.19 (1 H, t, J = 7.8 Hz, ArH); 7.38 (2 H, d, J = 7.5 Hz, ArH); 7.44 (2 H, t, J = 7.8 Hz, ArH); 7.55 (1 H, t, J = 7.2 Hz, ArH); 7.65 (1 H, s, ArH);7.71 (2 H, d, J = 7.2 Hz, ArH); 8.00 (1 H, s, 3-H) ppm; C21.33H21.66BrN2 O5 . Crystal Data of 6a. C21.33H21.66BrN2 O5 , M = 465.94, monoclinic, a = 10.890(2) Å, b = 16.371(4) Å, c = 11.951(3) Å, β = 94.556(18) °, V = 2123.9(8) Å3, T = 295(2), space group P21/n, Z = 4, μ(Mo Kα) = 1.970 mm-1. The final refinement parameters: R1 = 0.0524, wR2 = 0.1070 [for observed 3241 reflections with I > 2σ(I)]; R1 = 0.1298, wR2 = 0.1385 (for all independent 4916 reflections, Rint = 0.0387), S = 1.035. Largest diff. peak and hole 0.491 and –0.935 ēÅ-3.
References
- P Biginelli, P Gazz (1893) Synthesis of 3,4-Dihydropyrimidin-2(1H) Ones. Chim Ital 23(2): 360-416.
- Kappe CO (1993) 100 Years of the Biginelli Dihydropyrimidine Synthesis. Tetrahedron 49: 6937-6963.
- Kappe CO (2000) Biologically Active Dihydropyrimidones of the Biginelli-Type-A Literature Survey. Eur J Med Chem 35: 1043-1052.
- Wan JP, Liu Y (2010) Synthesis, pp. 3943.
- Nagarajaiah H, Mukhopadhyay A, Moorthy JN (2016) Tetrahedron Lett 57 (47): 5135-5296.
- Gartner M, Sunder Plassmann N, Seiler J, Utz M, Vernos I, et al. (2005) Chem Bio Chem 6: 1173.
- Russowsky D, Canto RFS, Sanches SAA, D`Oca MGM, Fatima A, et al. (2006) Bioorg Chem 34: 173.
- Tawfik HA, Bassyouni F, Gamal Eldeen AM, Abo Zeid MA, El Hamouly, et al. (2009) Tumor anti-initiating activity of some novel 3,4-dihydropyrimidinones. Pharmacol Rep 61(6): 1153-1162.
- Haggarty SJ, Mayer TU, Miyamoto DT, Fathi R, King RW, et al. (2000) Dissecting cellular processes using small molecules: identification of colchicine-like, taxol-like and other small molecules that perturb mitosis. Chem Biol 7(4): 275-286.
- Fewell SW, Smith CM, Lyon MA, Dumitrescu TP, Wipf P, et al. (2004) Small molecule modulators of endogenous and co-chaperone-stimulated Hsp70 ATPase activity. J Biol Chem 279(49):51131-51140.
- Karkola S, Lilienkampf A, Wahala K (2008) A 3D QSAR model of 17betaHSD1 inhibitors based on a thieno[2,3-d]pyrimidin-4(3H)-one core applying molecular dynamics simulations and ligand-protein docking. Chem Med Chem 3(3):461-472.
- Tozkoparan B, Yarim M, Saraç S, Ertan M, Kelicen P, et al. (2000) Studies on synthesis, chromatographic resolution, and anti-inflammatory activities of some 2-thioxo-1,2,3,4-tetrahydropyrimidines and their condensed derivatives. Arch Pharm Pharm 333(12): 415-420.
- Yadlapalli RK, Chourasia OP, Vemuri K, Sritharan M, Perali RS (2012) Synthesis and in vitro anticancer and antitubercular activity of diarylpyrazole ligated dihydropyrimidines possessing lipophilic carbamoyl group. Bioorg Med Chem 22(8): 2708-2711.
- Gein VL, Kholkin IV, Zamaraeva TM, Voronina EV, Vakhrin MI (2012) Pharm Chem J 46: 114.
- Gein VL, Fedotov, A Yu, Zamaraeva TM, Bobyleva AA, Kasimova NN, et al. (2012) Pharm Chem J 46: 720.
- Zamaraeva TM, Odegova TF, Fedotov A Yu, Tomilov MV, Gein VL, Slepukhin PA (2010) Synthesis and antimicrobial activity of 6-aryl-3,4-dimethyl-Nphenyl-2-oxo-1,2,3,6-tetrahydropyrimidine-5-carboxamides. Russ J Gen Chem 84(10): 1950-1952.
- Godhani DR, Dobariya PB, Jogel AA, Sanghani AM, Mehta JP (2014) Med Chem Res 23: 2417.
- Kushnir OV, Voloshchuk ON, Efteneva RI, Marchenko MM, Vovk MV (2014) Pharm Chem J 48: 246.
- Zamanova AV, Kurbanova MM, Rzaeva IA, Farzaliev VM, Allakhverdiev MA (2010) Antioxidant properties of some 5-ethoxycarbonyl-substituted 3,4-dihydropyrimidin-2(1H)-ones (-thiones) and their derivatives. Russ J Appl Chem 83(2): 293-296.
- Ashok M, Holla BS, Kumari NS (2007) Convenient one pot synthesis of some novel derivatives of thiazolo[2,3-b]dihydropyrimidinone possessing 4-methylthiophenyl moiety and evaluation of their antibacterial and antifungal activities. Eur J Med Chem 42(3): 380-385.
- Atwal KS, Swanson BN, Unger SE, Floyd DM, Moreland S, et al. (1991) J Med Chem 34: 806.
- Marvaniya HM, Parikh PK, Sen DJ (2011) J Appl Pharm Sci 1(5): 109.
- Cho H, Ueda M, Shima K, Mizuno A, Hayashimatsu M, et al. (1989) J Med Chem 32: 2399.
- Atwal KS, Rovnyak GC, Kimball SD, Floyd DM, Moreland S, et al. (1990) Dihydropyrimidine calcium channel blockers. 2. 3-substituted-4-aryl1,4-dihydro-6-methyl-5-pyrimidinecarboxylic acid esters as potent mimics of dihydropyridines. J Med Chem 33(9): 2629-2635.
- Atwal KS, Swanson BN, Unger SE, Floyd DM, Moreland S, Hedberg A, O Reilly BC (1991) Dihydropyrimidine calcium channel blockers. 3. 3-Carbamoyl-4-aryl-1,2,3,4-tetrahydro-6-methyl-5- pyrimidinecarboxylic acid esters as orally effective antihypertensive agents. J Med Chem 34(2): 806-811.
- October N, Watermeyer ND, Yardley V, Egan TJ, Ncokazi K, et al. (2008) Med Chem Med 3: 1649.
- Chikhale RV, Bhole RP, Khedekar PB, Bhusari KP (2009) Synthesis and pharmacological investigation of 3-(substituted 1-phenylethanone)-4- (substituted phenyl)-1, 2, 3, 4-tetrahydropyrimidine-5-carboxylates. Eur J Med Chem 44(9): 3645-3653.
- Nagarathnam D, Miao SW, Lagu B, Chiu G, Fang J, et al. (1999) J Med Chem 42: 4764.
- Dhar TGM, Nagarathnam D, Marzabadi MR, Lagu B, Wong WC, et al. (1999) J Med Chem 42: 4778.
- Lagu B, Tian D, Nagarathnam D, Marzabadi MR, Wong WC, et al. (1999) Design and Synthesis of Novel α1a Adrenoceptor-Selective Antagonists. 3. Approaches To Eliminate Opioid Agonist Metabolites by Using Substituted Phenylpiperazine Side Chains. J Med Chem 42(23): 4794- 4803.
- Wong WC, Sun W, Lagu B, Tian D, Marzabadi MR, Zhang F, et al. (1999) Design and synthesis of novel alpha(1)(a) adrenoceptor-selective antagonists. 4. Structure-activity relationship in the dihydropyrimidine series. J Med Chem 42(23): 4804-4813.
- Gein VL, Krylova IV, Tsypliakova EP, Gaifullina AR, Varkentin LI, et al. (2009) Chem Heterocycl Compd 45(7): 829.

 Mini Review
Mini Review