Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Graziella Alebrant Mendes*, Miriam da Costa Oliveira, Maria Beatriz Kohek and Júlia Fernanda Semmelmman Pereira Lima
Received: February 16, 2019; Published: March 08, 2019
*Corresponding author: Graziella Alebrant Mendes, Postgraduate Program in Pathology, Porto Alegre, RS, Brazil
DOI: 10.26717/BJSTR.2019.15.002731
E-cadherin (ECAD) loss on cell surface is related to invasiveness, metastases and bad prognosis in several malignant tumors of epithelial origin. Pituitary adenomas are common and benign tumors; however, they can become aggressive and invade other tissues. The herein assessed research describe ECAD positivity in most pituitary adenomas, but the relation between ECAD and tumor invasiveness led to different results. Further research is needed in order to better understand the ECAD impact on tumor development.
Keywords: Pituitary Neoplasms; Cadherins; Cell Adhesion Molecules
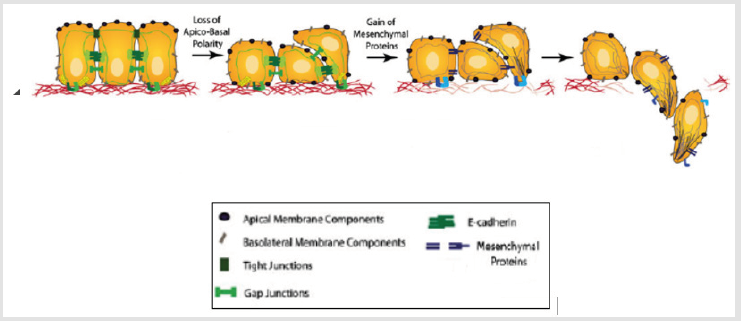
Cadherin of epithelial origin (ECAD) is a cell adhesion protein encoded by gene CDH1. Its loss on cell surface is related to invasiveness, metastases and bad prognosis in several malignant tumors of epithelial origin. ECAD has extracellular, transmembrane and cytoplasmic domain [1]. Several transcription factors are involved in epithelial-mesenchymal transition via E-cadherin repression, including SLUG, TWIST and SNAIL [2,3]. Epithelial-mesenchymal transition is an important process for embryonic development, in which cells lose their epithelial characteristics and gain mesenchymal properties. Such process is associated with cancer progression, with E-cadherin loss as the initial step of epithelial cell trans differentiation to the mesenchymal phenotype [4-7] (Figure 1).
Figure 1: Epithelial-mesenchymal transition. Epithelial cells lose apico-basal polarity and the tight junctions are dissolved during epithelial-mesenchymal transition. Epithelial proteins, such as E-cadherin, are suppressed and mesenchymal proteins, such as vimentin and fibronectin, are super-expressed. These cells become fusiform and able to move and invade the extracellular matrix (Adapted from Micalizzi et al. 2010 (8)).
Pituitary adenomas are common tumors, with estimated prevalence of 16.7%. Autopsy studies have reported their presence in 20% to 25% of cases; therefore, they account for 10% to 15% of primary intracranial tumors [9]. These tumors are benign; however, they can become aggressive and invade other tissues. Factors leading to these tumors’ growth and invasiveness are not completely understood [10], but some studies have shown that losses in adhesion protein expression can be associated with pituitary adenoma pathogenesis and contribute to tumor aggressiveness.
The herein assessed studies described the ECAD immunohistochemical positivity on most pituitary adenomas (Table 1). Kawamoto et al. [11] observed protein expression in all adenomas; Yamada et al. [12], found it in 39 out of 40 assessed cases; and Zhou et al. [13], in 93% of their sample. Other studies showed positivity in 52% to 73% of adenomas [14 -16]. The research conducted by Elston et al. [15] also showed ECAD expression in cell nucleus through an antibody applied to the ECAD’s cytoplasmic domain. It suggests that extracellular domain disruption and nucleus translocation are events that may contribute to pituitary adenoma pathogenesis. The research conducted by Fougner and his group included 80 acromegalic patients, of whom 41 (51.2%) had strong ECAD expression, 18 (22.5%) had intermediary expression and 21 (26.3%) had no expression on the cytoplasmic membrane. Elston et al. [15] assessed 10 GH producing adenomas, of whom 4 had strong to moderate immunohistochemical expression for ECAD, 2 had weak expression for it and 4 had none. They also identified positivity in 96.7% of the acromegalic patients [17]. Other authors reported lower ECAD expression on the cytoplasmic membrane of GH producing adenomas with prominent fibrous bodies than on other pituitary adenoma subtypes.
Table 1: Assessed studies about E-cadherin expression in pituitary adenomas based on the following variables: Author/year, sample, percentage of E-cadherin expression according to the used technique and its relation to tumor invasiveness.
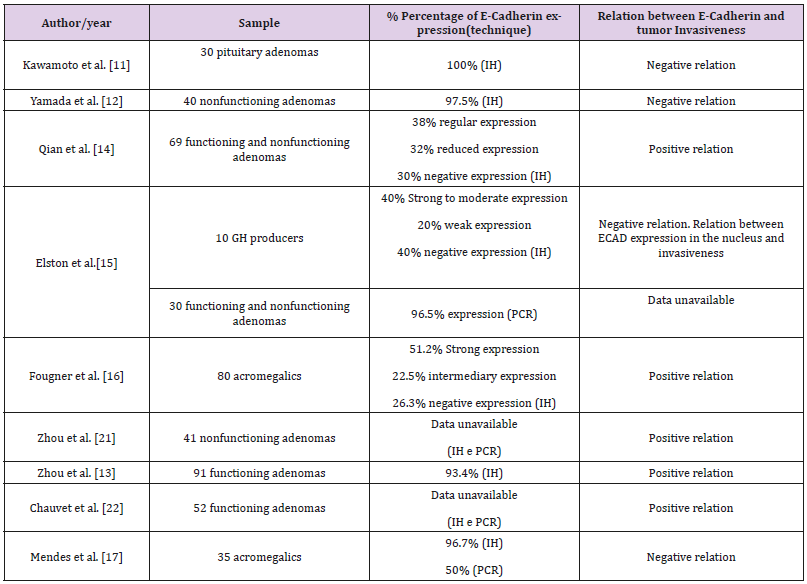
This outcome suggests that ECAD may be involved in the emergence of fibrous bodies [14,18-20]. There are few studies about ECAD gene expression in pituitary adenomas. The research conducted by Elston et al. [15] showed ECAD expression in 29 out of 30 assessed pituitary adenomas, whereas Mendes et al. [17] found it in 50% of their sample. According to research that have assessed the methylation of CDH1, up to 37% of pituitary adenomas have hypermethylation. The role of ECAD in pituitary adenoma invasiveness is not yet fully known, it remains inconclusive see Table 1. Four studies about the functioning and nonfunctioning pituitary adenomas did not find correlation between ECAD gene or immunohistochemical expression and tumor invasiveness [11- 17]. In the study conducted by Elston et al. [15], only ECAD nuclear expression was related to tumor invasiveness. On the other hand, Zhou et al. [21] reported that ECAD expression was significantly lower in invasive tumors in a research whose E-cadherin expression was assessed through immunohistochemistry and RT-PCR applied to nonfunctioning pituitary adenomas. Qian et al. [14] observed significant reduction in ECAD immunohistochemical expression in invasive tumors and macroadenomas.
The research conducted by Zhou et al. [13] with 91 pituitary adenomas showed significantly reduced ECAD immunohistochemical expression in invasive and recurrent tumors. Fougner et al. [16], in a study with acromegalic patients, reported negative relation among ECAD immunohistochemical expression, tumor invasiveness, tumor size and somatostatin analog response. The research conducted by Chauvet et al. [22] with GH producing adenomas reported significantly reduced ECAD gene and immunohistochemical expression in invasive tumors. Interestingly, the agreement between PCR and immunohistochemistry methods is poor because, although they are complementary techniques, each one analyzes different aspects of the cell. RT-PCR aims at verifying whether the gene that produces protein is activated through mRNA analysis, whereas immunohistochemistry verifies the presence of proteins. Presence of mutation in a small proportion of tumor cells, disorders in post-transcriptional and post-translational physiological regulations or contamination of tumor mRNA with normal tissue can affect the final results. In addition, one can take into consideration the heterogeneity of the tumor and the fact that the immunohistochemically analyzed tissue was not exactly the same tissue fragment assessed through RT-PCR [23].
Several biological mechanisms are related to the emergence and development of pituitary adenomas. Few researches assessed the presence of ECAD in these tumors and its impact on tumor invasiveness. The protein was identified in most tumors. Different results were observed in the assessed studies; thus, this outcome indicates that the pathogenesis of these tumors is complex. Further research are required to determinate the impact of ECAD on tumor development.


