Abstract
In-stent restenosis (ISR) is a potentially fatal complication which can be observed early or late after stent implantation. The patient-related factors associated with ISR include poor response to antiplatelet drugs, diabetes, malignancy, renal failure, and acute coronary syndrome. Higher rates of ISR are seen with increasing complexity of lesions. The underlying pathophysiology involves a complex interplay between platelets, leukocytes, coagulation-fibrinolysis system, and various growth factors and proinflammatory cytokines. We present a case of a patient who underwent in-stent restenosis in 21 stents despite being on adequate P2Y12 inhibition. This case highlights the role of coagulation pathways other than platelets in pathogenesis of ISR. We propose utilization of Thromboelastography in identifying patients at high thrombotic risk and consideration of using long-term oral anticoagulant therapy in addition to standard dual anti-platelet therapy in such patients.
Learning Objective: ISR involves coagulation pathways other than platelets. Thromboelastography can be utilized to identify patients with a thrombogenic phenotype in this population.
Keywords: In-stent restenosis; Thromboelastography; DAPT; Hypercoagulability
Introduction
In-stent restenosis (ISR) is an important complication observed after stent implantation with percutaneous coronary intervention (PCI) and is clinically defined as the onset of recurrent angina symptoms or objective evidence of myocardial ischemia. Angiographically, it is defined by ≥50% luminal diameter stenosis in the stented segment. The incidence of ISR varies with the type of stent used, patient and lesion characteristics, and procedural factors. The patient-related factors associated with late ISR (>30 days) include poor response to antiplatelet drugs, diabetes, malignancy, renal failure, and acute coronary syndrome (ACS) at presentation [1]. Higher rates of ISR are seen with increasing complexity of lesions. Data from meta-analysis and registries show a similar rate of early (<30 days) and late (>30 days but less than 1 year) ISR between bare-metal stents (BMS) and first-generation drug eluting stents (DES) [2,3]. With introduction of secondgeneration DES, rates of ISR have been reduced to 1.5% - 2.3% at 1 year [4,5]. The underlying pathophysiology of DES-ISR involves a complex interplay between platelets, leukocytes, coagulationfibrinolysis system, various growth factors and pro-inflammatory cytokines. We present a case of a patient who underwent ISR in 21 stents despite being on adequate P2Y12 inhibition. This case highlights the role of coagulation pathways other than platelets in pathogenesis of ISR.
Case Presentation
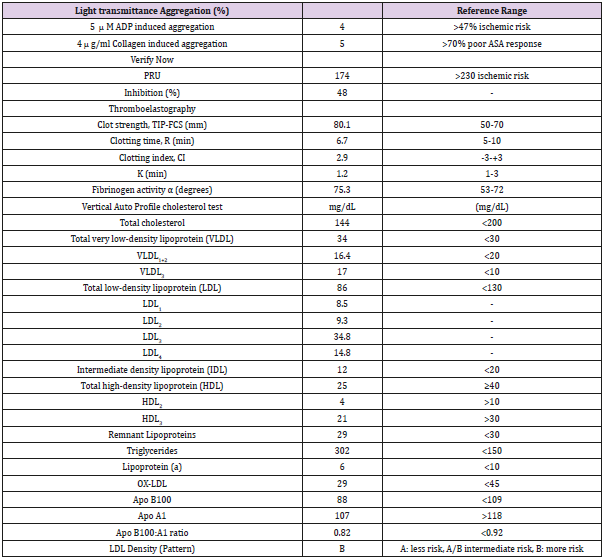
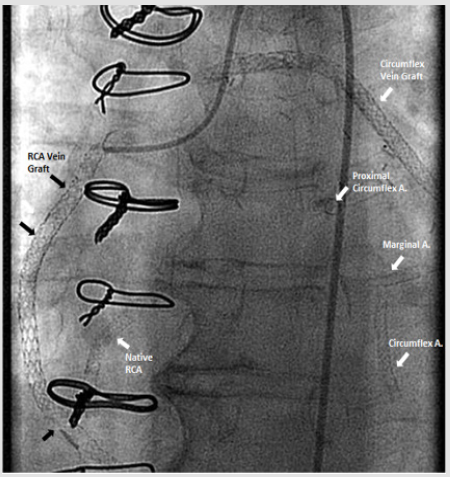
67-year-old Caucasian woman with past medical history of diabetes type 2, hypertension, peripheral vascular disease and severe coronary artery disease status post coronary artery bypass grafting 12 years ago (saphenous vein graft [SVG] to the first and third marginal branches of left circumflex artery, SVG to the posterior descending branch of the right coronary artery, left internal mammary artery to left anterior descending artery) with placement of a total of 21 stents (4 stents in SVG to right posterior descending graft; 12 stents in SVG to first and third marginal branch graft; 5 stents in native arteries), both BMS (n=5) and DES (n=16) over a course of 15 years, who presented with discomfort in jaw with minimal exertion for 3 days. The rest of review of systems was unremarkable. Her social history was remarkable for an active 30 pack-year smoking history and medications included aspirin, clopidogrel, insulin, atorvastatin, and isosorbide-mononitrate. Physical examination was unremarkable, and EKG showed an old right bundle branch block and old Q waves in lead III. Her laboratory data including complete blood count, basic metabolic panel, and troponins were within normal range. She was scheduled for cardiac catheterization and underwent additional testing with Thromboelastography (TEG), Verify Now P2Y12 point-of care assay and Vertical Auto Profile (VAP) cholesterol test to assess physical characteristics of platelet-fibrin clot, platelet reactivity and an extended lipid panel prior to catheterization (Table 1). She was found to be a clopidogrel responder with adequate P2Y12 inhibition and hypercoagulable. VAP cholesterol panel was significant for LDL cholesterol density pattern B. Coronary angiography was performed which showed an in-stent restenosis of the circumflex graft right above the anastomosis to distal marginal which was stented with a 3.0 x 15 mm resolute DES (Figure 1).
Table 1: Verify now, Platelet aggregation, Thromboelastography and VAP Indices.
Note: PRU=Platelet reactivity units, TIP-FCS= thrombin induced platelet-fibrin-clot strength, CI= Coagulation index, K= the time from R to reach a 20 mm of clot strength, VLDL=very low density lipoprotein, LDL=low density lipoprotein, IDL= Intermediate density lipoprotein, HDL=high density lipoprotein, ox-DLD= oxidized LDL, APO= apolipoprotein.
Discussion
This case describes a patient who despite being on adequate P2Y12 inhibition had multiple episodes of ISR. The current management guidelines for ISR with DES recommend using dual antiplatelet therapy (DAPT) with aspirin and P2Y12 inhibitors (clopidogrel, prasugrel or ticagrelor) for at least 1 year with the aim of preventing thrombotic events, particularly stent thrombosis. Although DAPT reduces the occurrence of ischemic events, its efficacy in reducing ISR remains uncertain [6]. Several factors have been attributed to increasing the risk of ISR in patients including procedural, angiographic and clinical factors. Mechanical endothelial injury during percutaneous coronary intervention has been linked to platelet activation, inflammation and occurrence of thrombotic events. The surface receptors of activated platelets facilitate attachment of leukocytes to the area of injury and further promote release of growth factors and cytokines which mediate hyperplasia and neo-intimal growth. Clinical factors which can favor a shift to thrombosis in the thrombosis-fibrinolysis equilibrium include a preexisting hypercoagulable state. As noted by our group in a prior study, TIP-FCS >67 mm is associated with a thrombotic phenotype predictive of restenosis, which was observed in the case discussed [7]. An alternate explanation of the observed hypercoagulability could be from the high number of stents implanted as opposed to intrinsic patient-related factors. LDL sub-patterns have also been shown to be associated with progression of atherosclerosis, vascular inflammation and neointimal hyperplasia [8]. A Type B pattern was observed in this case report indicating a higher risk of adverse events. In addition to the patient-related factors, recent studies have shown that patients with ISR do poorly with SVG grafts mid-term with early graft failure [9].
The proposed hypotheses include reduced production of nitric oxide in venous grafts (and not arterial) and the differential expression of thrombin receptors between arterial and venous grafts. It has been suggested that these patients would have better outcomes with arterial grafts. The ability to predict risk of restenosis can potentially lead to a more selective use of stents as well as a more aggressive, personalized antithrombotic therapy for improved clinical outcomes. Albeit the lack of data, it appears that Thromboelastography (TEG) and LDL subtypes might aid in guiding further characterization of these patients. Currently, the European Medicines Agency has approved the use of rivaroxaban to prevent atherothrombotic events after an acute coronary syndrome event in 2013 based on the results of ATLAS-ACS TIMI-51 trial. This trial randomized 15,526 patients to receive rivaroxaban or placebo in addition to DAPT and showed a significant reduction in the composite of death from cardiovascular causes, myocardial infarction, or stroke by 8.9% versus 10.7% with placebo (p=0.008) without a significant increase in fatal bleeding especially with 2.5 mg two-times a day dosing regimen [10]. We propose that such patients (with an increased thrombogenicity) should be further studied and characterized to further the personalization of the management of coronary artery disease. In conclusion, this case highlights the role of coagulation pathways other than platelets in pathogenesis of ISR. Also, we propose using TEG to identify patients with a thrombogenic phenotype that puts them at a higher risk of thrombosis. Further clinical outcome studies with biomarker assessments as described above are needed.
Conflicts of Interest
Conflicts of interest are as follows: Thromboelastography supplies were provided by Haemonetics® Corp. VAP© testing was provided by Atherotech® Inc. Dr. Gurbel reports serving as a consultant for Daiichi Sankyo, Sankyo, Lilly, Bayer, AstraZeneca, Accumetrics, Merck, Medtronic, CSL, and Haemonetics; receiving grants from the National Institutes of Health, Daiichi Sankyo/ Lilly, CSL, AstraZeneca, Harvard Clinical Research Institute, Bayer, Haemonetics, Duke Clinical Research Institute, Sinnowa, Coramed and Accumetrics; receiving honoraria and payment for lectures, consultations, including service on speakers’ bureaus from Daiichi Sankyo/ Lilly, Bayer, Merck and Boehringer Ingleheim; Dr. Gurbel is holding stock or stock options in Merck, Medtronic, and Pfizer; and holding patents in the area of personalized antiplatelet therapy and interventional cardiology. Other authors report no conflicts of interest. No grants, scholarships, or funding was received for this manuscript.
References
- Jaffe R, Strauss BH (2007) Late and very late thrombosis of drug-eluting stents: evolving concepts and perspectives. J Am Coll Cardiol 10(50): 119-127.
- Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schömig A, et al. (2007) Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet 370(9591): 937-948.
- Lagerqvist B, James SK, Stenestrand U, Lindbäck J, Nilsson T, et al. (2007) SCAAR Study Group. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med 356(10): 1009-1019.
- Serruys PW, Silber S, Garg S, van Geuns RJ, Richardt G, et al. (2010) Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med 363: 136-146.
- Cassese S, Ndrepepa G, King LA, Tada T, Fusaro M, et al. (2012) Two zotarolimus-eluting stent generations: a meta-analysis of 12 randomised trials versus other limus-eluting stents and an adjusted indirect comparison. Heart 98: 1632-1640.
- Steinhubl SR, Ellis SG, Wolski K, Lincoff AM, Topol EJ (2001) Ticlopidine pretreatment before coronary stenting is associated with sustained decrease in adverse cardiac events: data from the Evaluation of Platelet IIb/IIIa Inhibitor for Stenting (EPISTENT) Trial. Circulation 103(10): 1403-1409.
- Bliden KP, Tantry US, Gesheff MG, Franzese CJ, Pandya S, Toth PP, et al. (2016) Thrombin-Induced Platelet-Fibrin Clot Strength Identified by Thrombelastography: A Novel Prothrombotic Marker of Coronary Artery Stent Restenosis. J Interv Cardiol 29(2): 168-178.
- Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, et al. (1988) Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA 260(13): 1917-1921.
- Gaudino M, Luciani N, Glieca F, Cellini C, Pragliola C, Trani C, et al. (2006) Patients with in-stent restenosis have an increased risk of mid-term venous graft failure. Ann Thorac Surg 82(3): 802-804.
- Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, et al. (2012) Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 366(1): 9-19.

 Case Report
Case Report