Abstract
The review integrates and systematically describes the data on the synthesis of three-, four-, five-, six-, and eight-membered cyclic and acyclic amino-peroxides.
Keywords:Aza-Peroxide Compounds; Dioxaziridines; Dioxazetidines; Dioxazetes; Dioxadiazetidines; 1,2,4-Dioxazolidine; Dioxatetrahydropyridazines; Endoperoxides; Ozonolysis; Tetraoxazaspiroalkanes; Tetraoxazaspiroalkanes; Acyclic Aminodiperoxides; Catalysis
Introduction
The enhanced interest in organic peroxide compounds is caused by their broad scope of applications, first of all, in chemical and pharmaceutical industry and in laboratory practice. A real breakthrough in the synthesis of peroxide compounds was made after the discovery of the antimalarial activity of peroxides, which is utilized in highly efficient medicinal drugs (artemisinin, artesunate, artemether, dihydroartemisinin). The advances in the peroxide chemistry and pharmacology stimulated the research related to the synthesis of heteroatom-containing peroxides. Out of heteroatomcontaining peroxides, attention of researchers is focused on aminoperoxides. This enhanced interest in aza-peroxides is attributable to the fact that many natural compounds (verruculogen, dioxetanone) and antimalarial agents (RKA182 and OZ439) contain aza-peroxy moieties in the molecules. The extensive biological activity of nitrogen-containing peroxides promoted active research on the development of synthetic routes to new classes of acyclic and cyclic amino-peroxides.
It is noteworthy that a compound containing both a nitrogen atom and a peroxide group in the molecule was first mentioned in the world literature back 1900 [1]. Despite more than a 100- year history, amino-peroxides remain poorly studied because of complicated synthesis and small number of available preparation methods. In view of the high practical significance of fundamental and applied research dealing with the synthesis and use of aminoperoxides and extensive interest of researchers in this promising and intriguing field of chemistry, in the present review, we attempted to give a critical account of the achievements of both foreign and Russian researchers engaged in the synthesis and studies of the properties of three-, four-, five-, six-, and eight-membered cyclic and acyclic amino-peroxides with the goal of integrating published results.
Three-Membered Aza-Peroxides
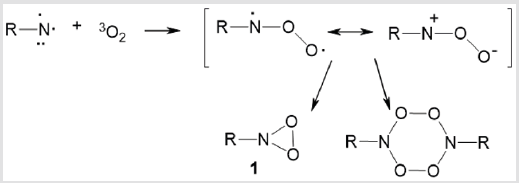
The simplest cyclic peroxide derivatives are composed of a 3-membered ring consisting of one nitrogen and two oxygen atoms. These dioxaziridine rings can be formed both from nitro compounds [RN (O) O] and from nitroso O-oxides (RNOO) (Scheme 1). Dioxaziridines 1 (aza-dioxiranes) have not been isolated in a pure state, but have been identified as unstable intermediates in solutions in 2-methyltetrahydrofuran at 77 K [2] or acetonitrile at 298 K [3].
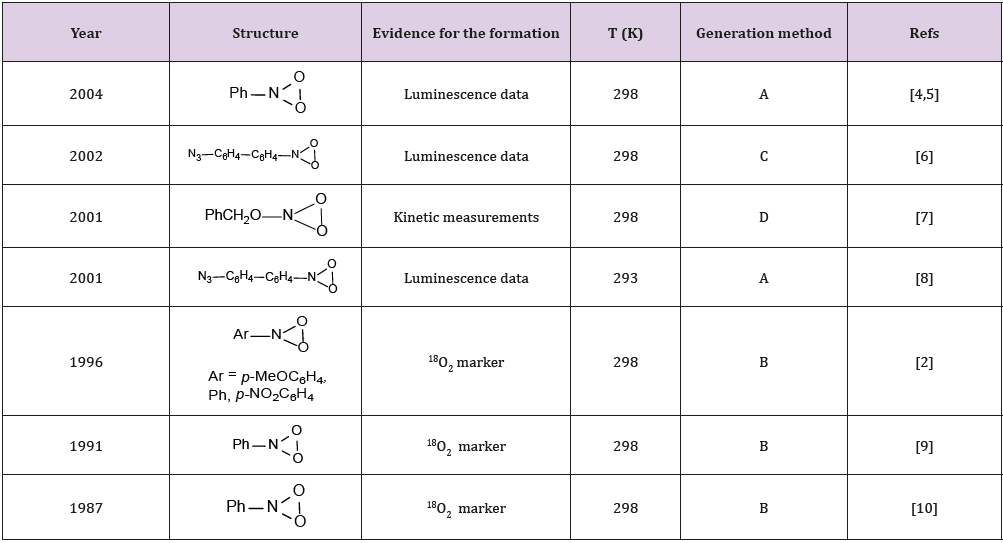
Dioxaziridines are unstable at room temperature. Most often, they are detected by UV spectroscopy. The key methods used to generate dioxaziridines are photooxidation of aryl azides and О-substituted diazeniumdiolates according to Scheme 1. The main representatives of dioxaziridines and methods for their generation are summarized in Table 1.
Table 1: Generation of dioxaziridines 1 (RNO2 dioxiranes).
Note: А, Matrix isolation upon photooxidation of ArN3; B, photooxidation of ArN3 at room temperature in solution; C, substrates adsorbed on natural rubber; D, photooxidation of diazoniumdiolate in solution at room temperature.
Four-Membered Aza-Peroxides
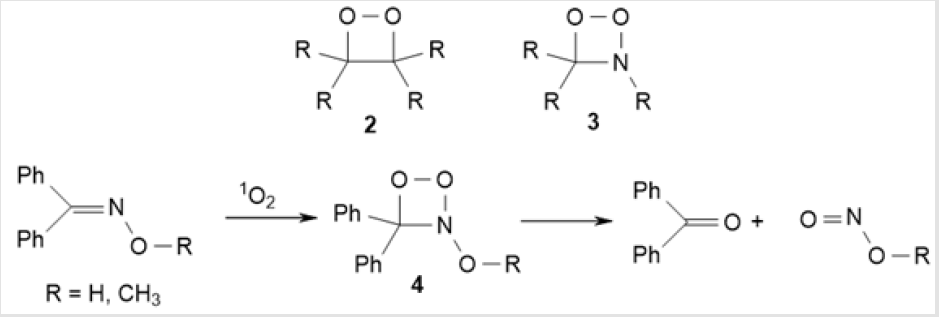
Four-membered aza-peroxides are represented by three types of compounds: dioxazetidines, dioxazetes, and dioxadiazetidines. 1,2-Dioxetanes 2 without nitrogen atom in the ring are among the most readily accessible four-membered peroxide heterocycles, which are synthesized by treatment of alkenes with singlet oxygen [11,12]. Adamantyl- and alkoxy-substituted 1,2-dioxetanes are most stable, whereas other derivatives easily decompose to carbonyl compounds [13]. A similar decomposition pathway should be expected for 1,2,3-dioxazetidines 3, which are generated upon the reaction of singlet oxygen with imines [14,15]. This reaction was first studied in relation to treatment of benzophenone oxime with singlet oxygen to give the oximate anion and О-methyl ether 4 (Scheme 2) [16].
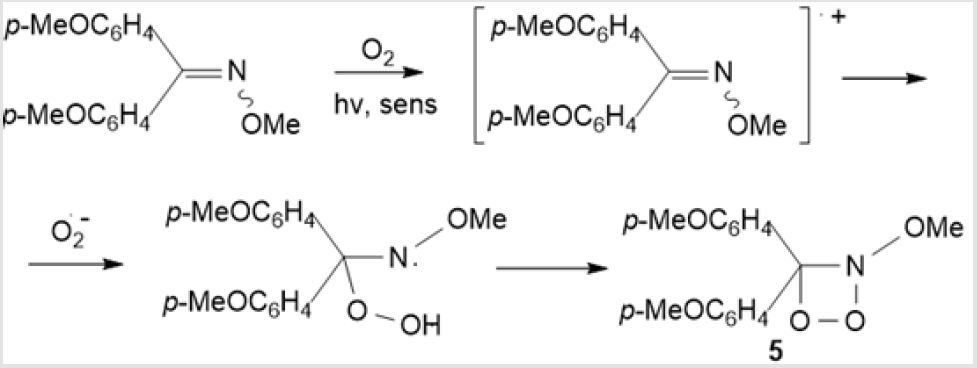
However, C=N containing compounds are not always able to be converted by this photooxidation mechanism. The photooxidation of acyclic ketoximes, aromatic aldoximes and ketoximes, and α-oximino ketones yields the corresponding aldehydes resulting from the competing oxidation of the С=С bond [17]. Amidoximes behave in a similar way, but in this case, the anionic species react with oxygen to give nitriles and amides (via intermediate acyclic peroxide derivatives) rather than 1,2,3-dioxazetidines [18]. Another reaction pathway to the intermediate formation of 1,2,3-dioxazetidine 5 involves, instead of singlet oxygen, UV-induced photooxidation of N-methoxy-4-methoxyphenyl-4’-methylphenylmethanimine in the presence of 9,10-dicyanoanthracene as a photosensitizer (Scheme 3) [19,20]. The 1,2,3-dioxazetidine 5 thus formed decomposes to give diaryl ketone and methyl nitrite with photoisomerization of the C=N double bond [19].
Scheme 3: UV photooxidation of N-methoxy-4-methoxyphenyl-4’-methylphenylmethanimine with the photosensitizer 9,10-dicyanoanthracene.
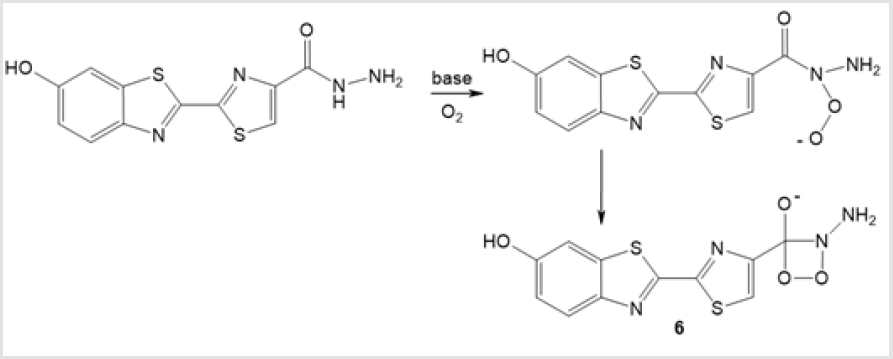
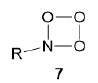
1,2,3-Dioxazetidine 6 is formed as an intermediate upon luminescence of acyl hydrazide based on 5-methyldehydroluciferin in the presence of a strong base (Scheme 4) [21]. Nitroso compounds such as N,N-dimethyl-4-nitrosoaniline possess bleaching properties owing to the reaction of singlet oxygen with imidazole or histidine [22], which involves the step of endoperoxide formation [23,24]. The authors assume the intermediate formation of trioxazetidine 7 upon the [2 + 2] -cycloaddition of nitroso groups.
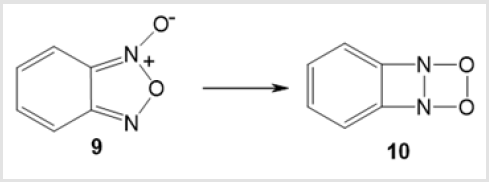
1,2,3,4-Dioxadiazetidines 8 are dimers of nitroso compounds. They are also products of [2 + 2]-cycloaddition of azo compounds and oxygen. Nevertheless, they have not yet been characterized. An example of intermediate formation of energetic 1,2,3,4-dioxadiazetidine 10 upon isomerization of benzofurazan 1-oxide (benzofuroxan) 9 has been reported [25] (Scheme 5).
Five-Membered Aza-Peroxides
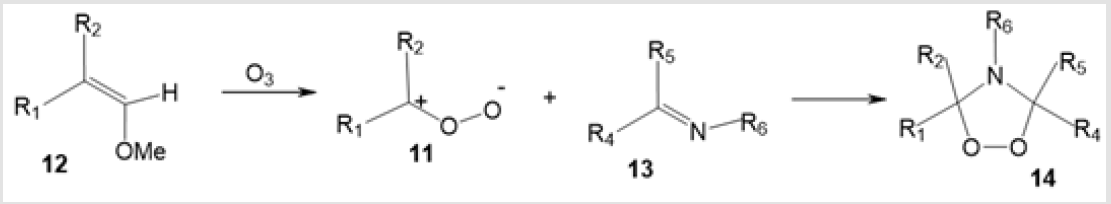
Ozonolytic Methods for the Synthesis of Five-Membered Aza-Peroxides: The most efficient method for the preparation of cyclic aza-peroxides is the ozone oxidation of the olefinic bond in the presence of nitrogen-containing compounds. For instance, ozone treatment of vinyl ethers 12 in the presence of imines [26] leads to the [3+2]-cycloaddition of carbonyl oxides 11 generated upon ozonation to appropriate imines 13 to give 1,2,4-dioxazolidine five-membered ring 14 (Scheme 6).
Scheme 6: Synthesis of 1,2,4-dioxazolidines by the ozonolysis of vinyl ethers in the presence of imines.
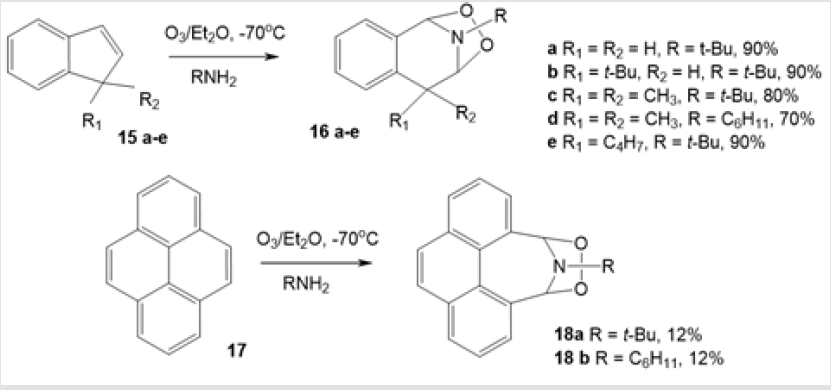
It is noteworthy that the reactivity of intermediate carbonyl oxide 11 in this reaction depends on the steric factors involved in its approach to the imine, which are normally determined by the carbonyl oxide structure. The reaction is stereoselective in most cases: out of the 26 compounds obtained by this method, only seven products were formed as isomer mixtures [26]. The ozone oxidation of indene 15а-е and pyrene 17 derivatives in the presence of primary amines afforded bicyclic 1,2,4-dioxazolidines [27]. Treatment of a mixture of substituted indenes 15a-e and primary amines with ozone at –70°С gave 1,2,4-dioxazolidine derivatives 16a-e in high yields. Ozonolysis of pyrene 17 under similar conditions resulted in the synthesis of cyclic amino-peroxides 18a,b, but the conversion was low (Scheme 7). Various primary amines such as cyclohexylamine, benzylamine, aniline, and tertbutylamine can serve as the nitrogen cross-components. Unlike primary amines, secondary amines cannot be used in this reaction, as they are readily oxidized themselves.
Scheme 7: Synthesis of 1.2.4-dioxazolidines derivatives by the ozonolysis of indenes in the presence primery amines.
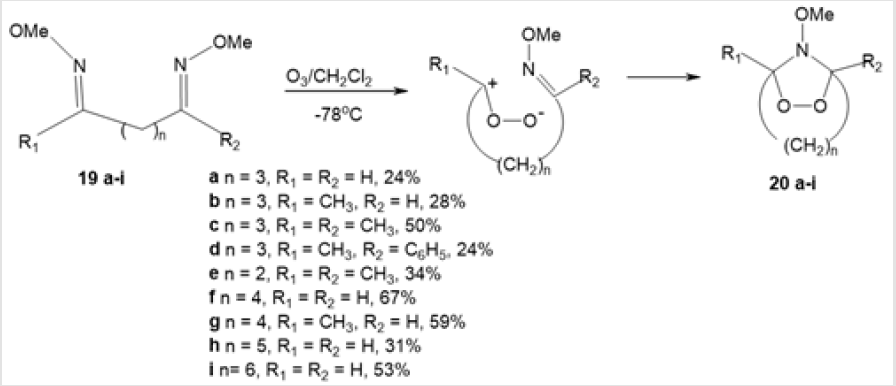
An efficient method for the formation of N-methoxy-substituted 1,2,4-dioxazolidine moiety is ozonolysis of dimethyldioximes of 1,4- and 1,5-diarbonyl compounds [28]. This one-step method is characterized by good conversion and stereoselectivity. While developing studies on ozonolysis of vinyl ethers in the presence of imines, K. Griesbaum and-co-workers investigated ozonolysis in the absence of carbonyl oxide acceptors. The reaction of dimethyldioximes 19а-е with ozone in an inert solvent afforded N-methoxy- 1,2,4-dioxazolidine derivatives 20а-е (Scheme 8). Relying on these data, Kang-Ryul Lee and co-workers [29] accomplished ozonation of аcyclic O-methylated dioximes 19f-i with n = 4–6 and aromatic O-methylated dioximes 21a and 21b (Scheme 9). Ozonolysis of 19f-i in dichloromethane at -78°С furnished the corresponding bicyclic 1,2,4-dioxazolidines 20f-i in 67, 59, 31, and 53% yields, respectively.
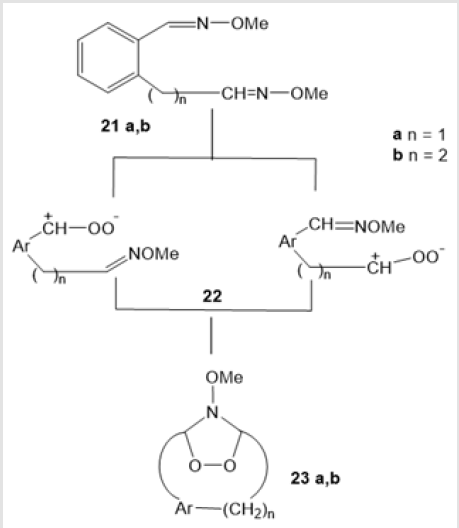
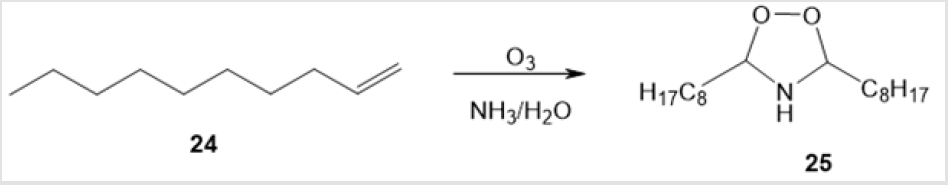
The ozonolysis [29] of aromatic О-methylated dioximes 21a and 21b in dichloromethane at -78°С gave rise to aromatic 1,2,4-dioxazolidines 23a and 23b via intermediates 22 in 65 and 35% yields. Yet another example of ozonolytic preparation of 1,2,4-dioxazolidines is ozonation of 1-decene 24 in a homogeneous system containing ammonia and water (Scheme 10) [30]. The amino-peroxide 25 thus formed was unstable and decomposed in air.
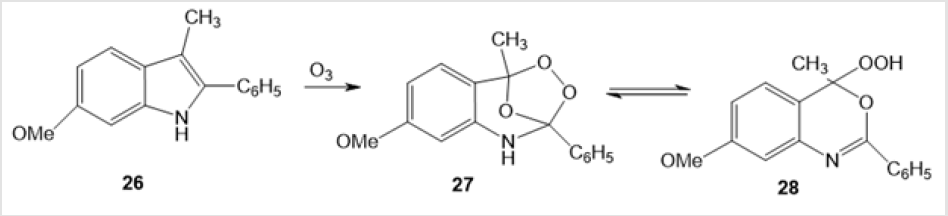
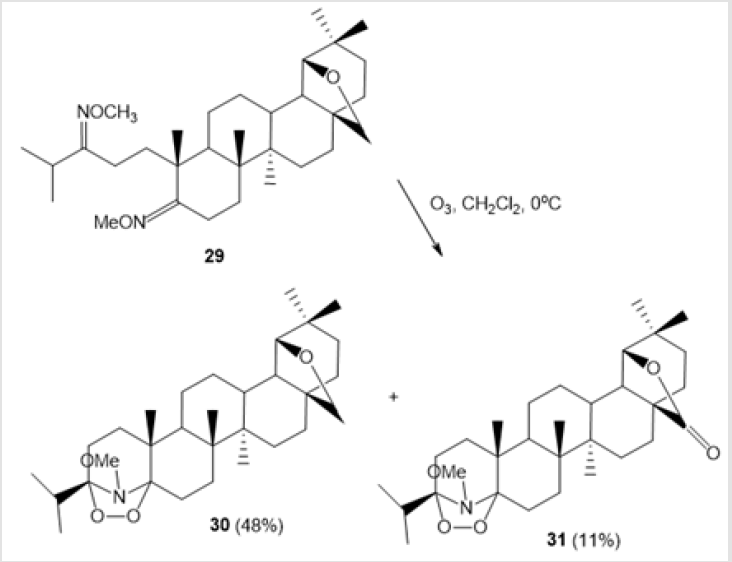
The ozonation of 3-methyl-6-methoxy-2-phenylindole (2-phenylskatole) 26 leads to 2-phenylbenzoxazine hydroperoxide able to exist as two tautomers occurring in equilibrium, ozonide 27 and hydroperoxide 28 (Scheme 11) [31]. The first synthesis of triterpenoid amino-peroxide with 1,2,4-dioxazolidine moiety by ozonation of 3,5-seco-18α-oleane 3,5-bismethyl dioxime 29 in CH2Cl2 at 0°С has been discussed in the literature [32]. The reaction is stereoselective and gives only one (3S,5S)-diastereomer of 19β,28-epoxy-А-neo-3,5-seco-3S,5S-dioxy-3,5-N-methoxyazo- 18α-oleane 30 (48%). 19β,28-Epoxy-28-oxo-А-neo-3,5-seco-3S,5Sdioxy- 3,5-N-methoxyazo-18α-oleane 31 is formed as a side product in 11% yield (Scheme 12).
Scheme 12: Synthesis of triterpenoid-based 1,2,4-dioxazolidines by ozonolysis of allobetulin derivatives.
Photochemical Methods for the Synthesis of Five- Membered Aza-Peroxides
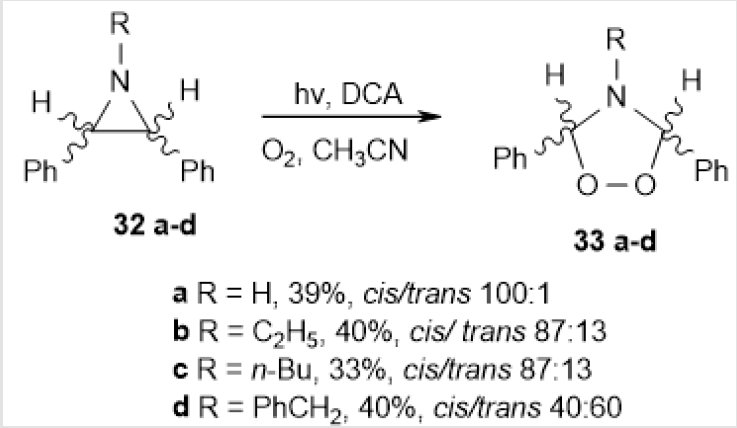
1,2,4-Dioxazolidines can be prepared by photochemical oxidation with singlet oxygen. Using this approach, 1,2,4-dioxazolidines 33a-d were synthesized for the first time by photooxidation of аziridines 32a-d in dry acetonitrile in the presence of 9,10-dicyanoanthracene (Scheme 13) [33-35]. The reaction is stereoselective if no steric restrictions are present at the nitrogen atom. The stereochemistry of the reaction products does not depend on cis-/trans- positions of substituents at the neighboring carbon atoms in the starting compounds.
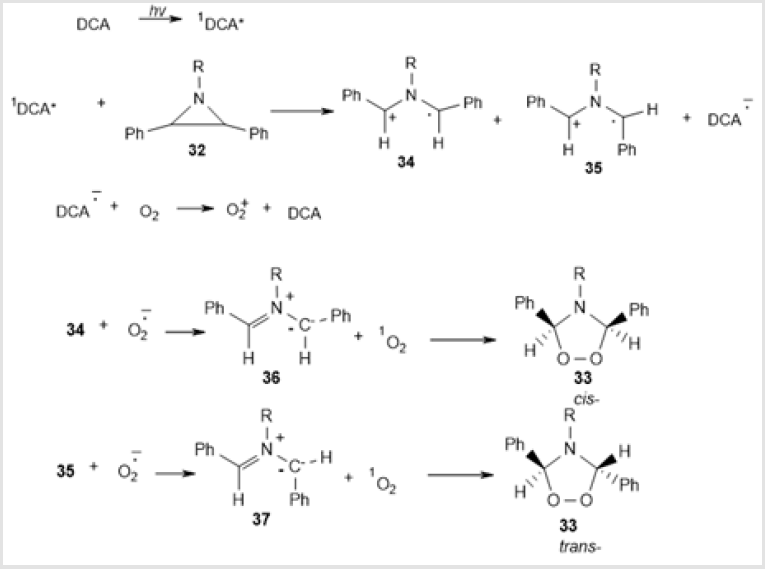
The presumptive scheme of photochemical transformation of аziridines 32 into 1,2,4-dioxazolidines 33 includes аziridine ring opening under the action of singlet 9,10-dicyanoanthracene to give radical cations 34 and 35 in which the cis-configuration is the most stable. Then electron transfer takes place, resulting in azomethines 36 and 37, which undergo 1,3-dipolar cycloaddition with singlet oxygen to give 1,2,4-dioxazolidine compounds 33a-d (Scheme 14). If bulky substituents are present, cis-configuration of the intermediate radical cation 34 becomes unfavorable, which results in the formation of a mixture of cis- and trans- amino-peroxides. Therefore, the reaction is stereoselective only in the former case [33-35].
Scheme 14: Proposed mechanism involving addition of singlet oxygen to intermediate azomethine ylides.
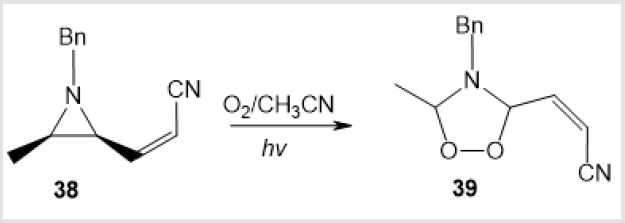
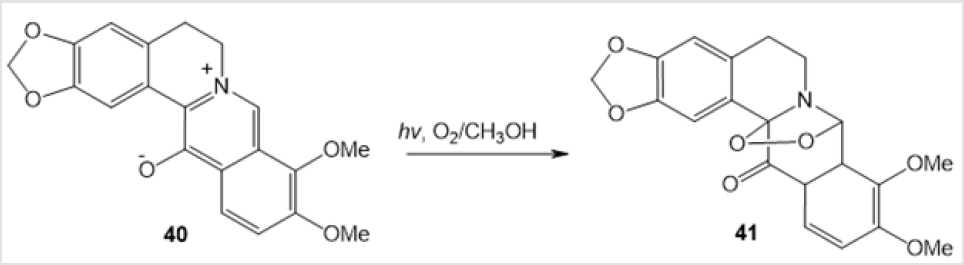
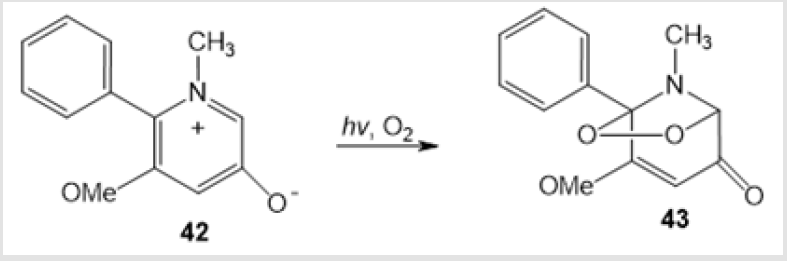
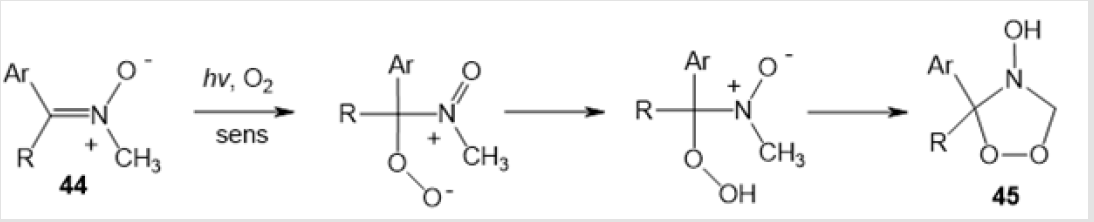
An example of photochemical synthesis of (Z)-(4-benzyl- 5-methyl-1,2,4-dioxazolidin-3-yl) acrylonitrile 39 in 24% yield by irradiation of cis-methylаziridine in acetonitrile has been reported (Scheme 15) [36]. The visible light irradiation of the alkaloid derivative 13-oxidoberberine 40 in the presence of Bengal Rose dye afforded amino-peroxide 41 in 42% yield [37] (Scheme 16). Y. Tamura and co-workers used photooxidation of 5-methoxy-1-methyl-6- phenylpyridin-3-oate 42 to perform a similar transformation giving 1,3-dipolar cycloadduct 43 (Scheme 17) [38]. 4-Hydroxy-1,2,4-dioxazolidine 45 was formed only as an intermediate in the photooxidation of N-methylnitrone 44 (Scheme 18) [21].
Miscellaneous Methods for the Preparation of Five- Membered Aza-Peroxides
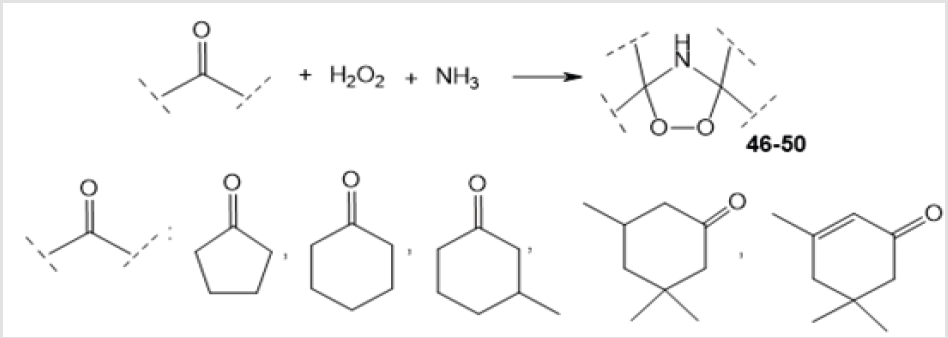
The ozonation and photooxidation of appropriate unsaturated compounds are the major methods for the preparation of the 1,2,4-dioxazolidine moiety. However, an amino-peroxide moiety can also be generated using some other approaches. A simple method for the synthesis of 1,2,4-dioxazolidines 46-50 in moderate yields is the Mannich reaction of alicyclic ketone with hydrogen peroxide and ammonia (Scheme 19) [39].
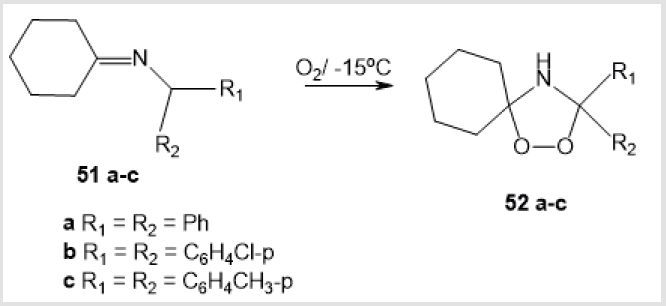
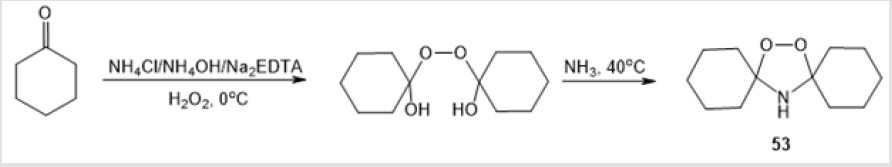
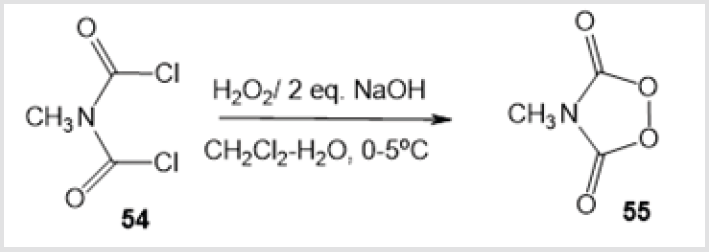
A synthetic route to 1,2,4-dioxazolidines 52а-с (formed in 60-80% yield) via oxidation of imines 51а-с in a petroleum ether– benzene solution with air oxygen at –15 to –20°С was discussed (Scheme 20) [40]. 1,1’-Peroxycyclohexylamine 53, which is a key intermediate in the production of Nylon-6 polymer [41], was also prepared by treatment of cyclohexanone with H2O2 in the presence of NH4Cl/NH4OH and Na2 EDTA followed by treatment with NH3 (Scheme 21) [42]. Mention should be made of the only method for the synthesis of 1,2,4-dioxazolidine-3,5-dione 55, a promising compound for industrial application as an initiator of free radical polymerization [43]. The reaction comprised treatment of N-methyliminodicarbonyl dichloride 54 with Н2О2 under alkaline conditions (Scheme 22).
Six-Membered Amino-Peroxides
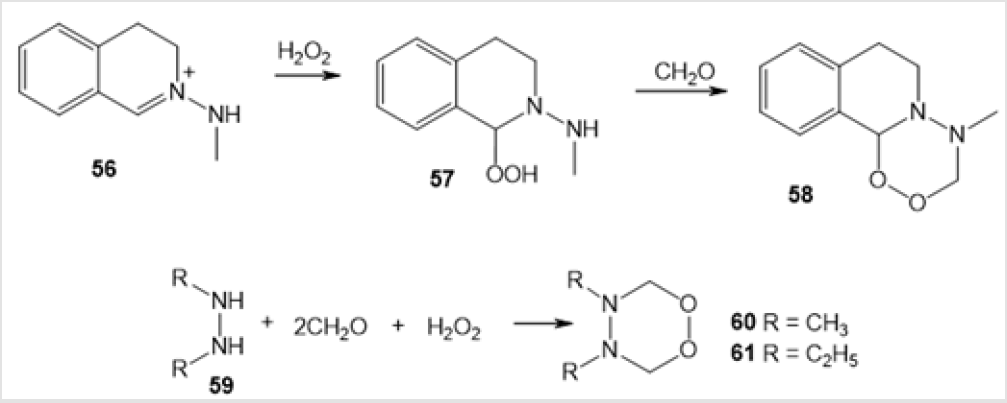
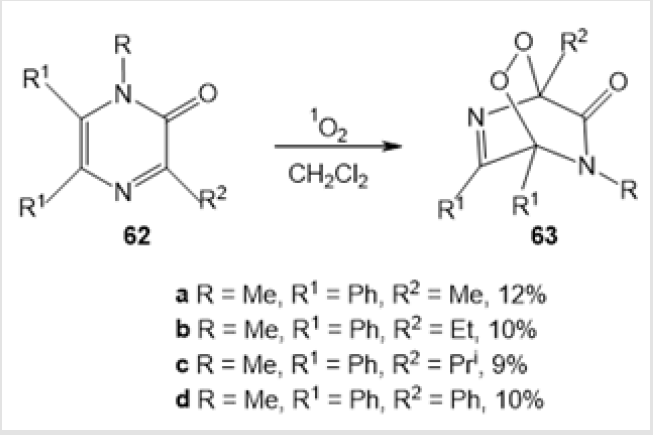
A synthetic route to 1,2-dioxatetrahydropyridazines 58, 60, and 61 [44] consisting in the reaction of compound 56 or 1,2-dialkylhydrazines 59 a,b with СН2О and Н2О2 was proposed (Scheme 23). The authors suggested the intermediate formation of hydroperoxide 57. The six-membered amino-peroxides reported in the literature are mainly endoperoxides prepared by photooxidation of nitrogen-containing ketones. Indeed, photooxidation of pyrazin- 2-ones 62 with singlet oxygen in the presence of Methylene Blue dye gave rise to epidioxypyrazin-2-ones 63 with a six-membered amino-peroxide ring in the molecule (Scheme 24) [45].
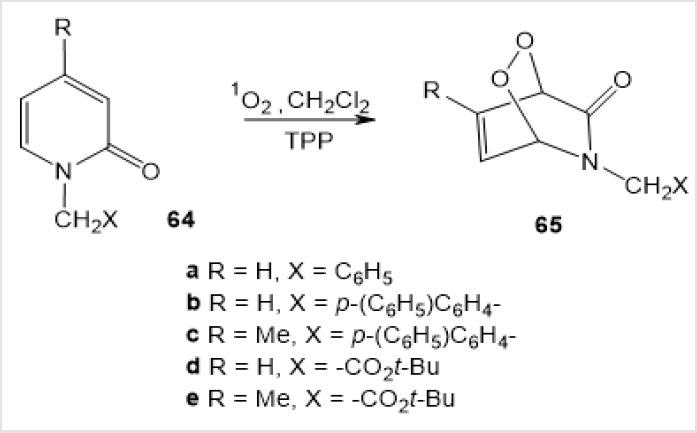
Irradiation of a solution of N-benzyl-2-pyridinones 64 in dichloromethane with a Na-lamp (940 W) in the presence of a catalytic amount of Tetraphenylporphin (TPP) in an oxygen atmosphere at -78°С for 2 h yielded exclusively 1,4-endoperoxides 65 (Scheme 25) [46].
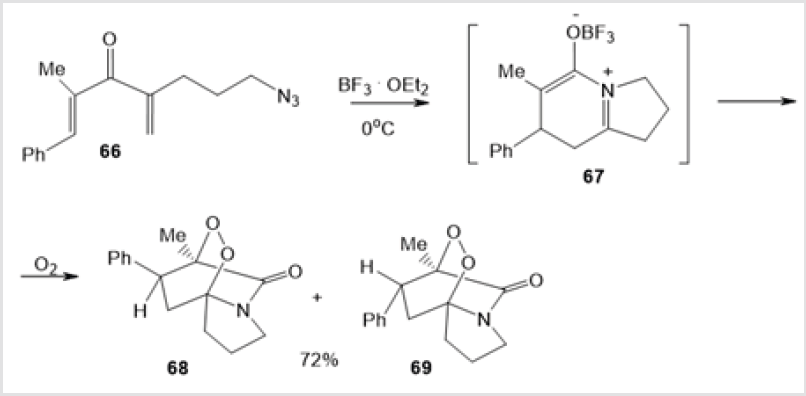
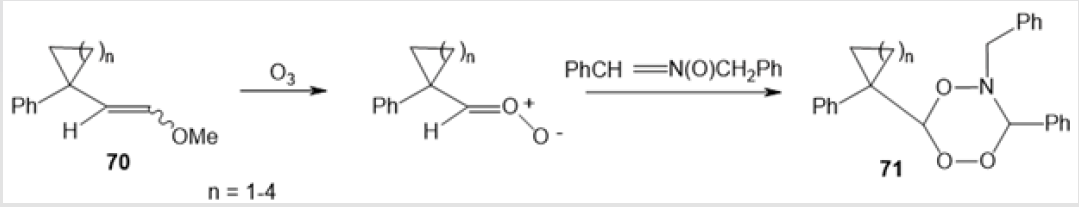
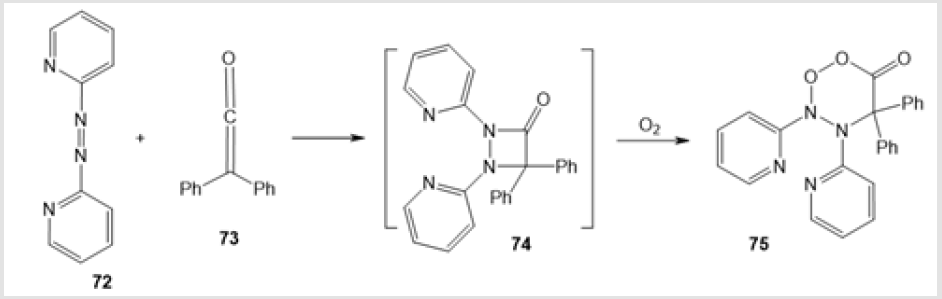
The oxidation of alkadienone 66 at 0оС catalyzed by boron trifluoride etherate gave an intermediate betaine complex with Lewis acid 67, which rearranged on further oxidation to a mixture of diastereomeric endoperoxides 68 and 69 in a total yield of 72% (Scheme 26) [47]. The ozonolysis of vinyl ethers 70 in the presence of α-phenyl-N-benzylnitrone afforded six-membered peroxides, 5-benzyl-6-phenyl-3-(1-phenylcycloalkyl)-5,6-dihydro-1,2,4,5- trioxazines 71, in 79-95% yields (Scheme 27) [48]. The cycloaddition of 2,2’-azodipyridine 72 to diphenyl ketene 73, obtained in situ, followed by self-oxidation of intermediate 1,2-diazetidinone 74 furnished target dihydro-5,5-diphenyl-3,4-bis(2-pyridyl)-1,2,3,4- dioxadiazin-6-one 75 (Scheme 28) [49].
Eight-Membered Aza-Peroxides
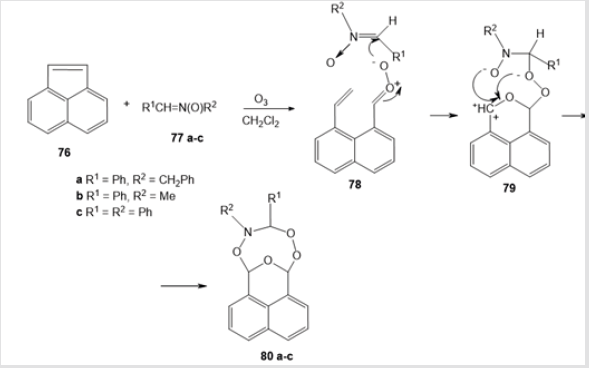
Ozonolysis of acenapthylene 76 in the presence of nitrones 77a-c is an efficient route to polycyclic amino-peroxides 80 а-с containing the dihydro-1,2,5,7,4-tetraoxazocine ring. The reaction proceeds as the [3+3+2]-cycloaddition between the carbonyl oxide moiety, nitrone, and aldehyde group via intermediates 78 and 79 (Scheme 29) [50]. The yields of eight-membered amino-peroxides 80a-c are 11, 30, and 45%, respectively (Figure 1).
Figure 1.

 Review Article
Review Article