Introduction
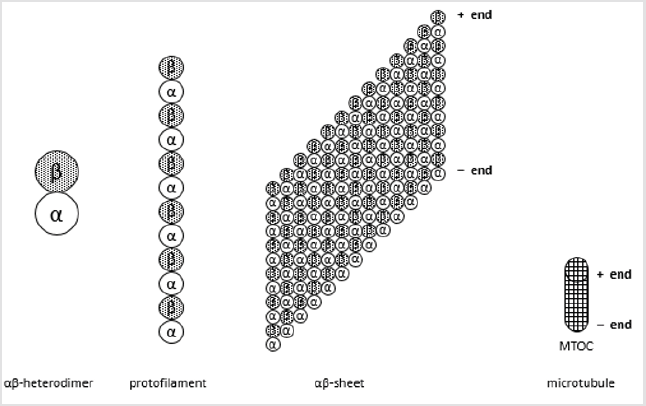
Microtubules from cytoskeleton are formed by tubulin dimers and they are responsible for the shape of eukaryotic cells. Tubulin dimers are formed by two globular proteins called α- and β-tubulin. Monomers from α- and β-tubulin share 40% amino acid identity and show similar 3D structure, with three functional domains: the carboxy-terminal domain (a binding domain for motor proteins), a nucleotide-binding domain (to bind GDP or GTP) and the taxol binding site (a domain that binds drugs and microtubule associated proteins) [1]. αβ-Heterodimers form polymers called protofilaments, where all heterodimers are bound one to the other following the same direction. In presence of Zn2+, tubulin is purified as a 13 aligned protofilaments sheet, where those protofilaments are one near the other and all α-tubulin are surrounded by β-tubulin. Nevertheless, in microtubules, this sheet is curved and fold to a hollow cylinder shape, the microtubule lattice. Microtubules are initiated at microtubule organizing centres (MTOC) that are usually near the cell nucleus. This is the minus-end, while the growing terminal is the plus-end (Figure 1).
Figure 1: Structure of microtubules.
Microtubules are polimers of 13 protofilaments, formed by polimers of tubulin. Tubulin is a dimer formed by α- and β-tubuline, two similar proteins with a carboxy-terminal domain, a nucleotide-binding domain (GDP or GTP) and a taxol binding site. α-Tubulin will be the minus-end and β-tubulin the plus-end. Growth and shrinkage are performed at the plus-end, as microtubules are bound to microtubule organizing centers (MTOC) at the minus-end.
Microtubules are not static structures. In fact, they alternate between polymerizing and shrinking phases, as in the socalled “dynamic instability” [2]. Polymerizing depends on the concentration of free αβ-heterodimer concentration and on the presence of GTP or GDP at the plus-end of the microtubule, as the growth is performed mainly at the plus-end. When the terminal plus-end β-tubulin contains GTP, further αβ-heterodimers are bound to the microtubule and a growth is observed. Nevertheless, when the terminal plus-end β-tubulin is bound to GDP, the structure of the microtubule changes by turning its structure outside down and a catastrophe is generated. Then, the tubulin heterodimers are released to media and the microtubules decrease their length. So, this signal is the main function of the nucleotide-binding domain: a GDP-cap in β-tubulin shows a shrinkage, whereas a GTP-cap a growth of microtubules. Whereas stabilization of microtubules through the nucleotide-binding domain cannot be externally controlled by some drugs, several compounds or proteins can interact on the carboxy-terminal domain or on the taxol binding site. In this paper, the effect of some tubulin modification or some compounds bound to tubulin are described for microtubules stabilization.
Effects of Modifications of Tubulin on Microtubule Stabilization
Carboxy-terminal domain contains amino acids that can be posttranslational modified. Those modifications can also correlate with microtubule stability [1]. Thus, by modifying enzymatically tubulin it is possible to stabilise microtubule. Examples on posttranscriptional modifications are acetylation on α-tubulin Lys 40, catalysed by histone deacetylase family member 6 (HDAC6) [3] and Sirtuin type 2 (Sirt2) [4]. By the other hand, acetyl transferase α-Tat1 (or Mec-17) unstabilizates microtubules [5,6].
The loss of C-terminal Tyr 451 in α-tubulin also stabilises microtubule. This tyrosine can be also enzymatically removed [7] and released [8]. Detyrosination also protects microtubules from depolymerizing activity of KIF2 or MCAK (kinesin-13 type motor proteins), thus increasing their stability [9,10]. Polyglutamylation was first described in brain [11], and it is not restricted to tubulin. It is observed on both α- and β-tubulin. Enzymes belonging to TTLlike (TTLL) family are responsible to catalyse polyglutamilation [12], whereas enzymes of the CCP family hydrolise C-terminal glutamate residues [13].
It is not clear whereas polyglutamilation stabilises or not microtubules. Polyglycylation is also performed on both α- and β-tubulin, with unclear results on stabilization of microtubules. TTLL family are the enzymes responsible of polyglycylation. In mammals, TTLL3 and TTLL8 are initiate glycylases, whereas TTLL10 is an elongate glycylase, although it seems that TTLL10 is inactive in humans, indicating that no elongation in glycine is observed in human [14]. Tubulin phosphorylation mainly takes place on Ser 172 in β-tubulin [15], catalysed by Cdk1. Polyamination is catalysed by transglutaminases, especially in Gln 15 of β-tubulin, stabilizing microtubules [16]. Tubulin can also palmitoylated [17], ubiquitinated [18], glycosylated [19], arginylated [20], methylated [21] and sumoylated [22], although no clear results have been presented on stabilization or not of microtubules.
Effect of Compounds Binding on Tubulin as Stabilizators
Some compounds are able to bind microtubules, by stabilizing or destabilizing its structure. As the GTP-cap is very important in microtubules for their growth, any drug interfering microtubule dynamics can be a good modulator of tubulin structure [23]. Among the most known compounds that stabilise microtubules structure, paclitaxel or taxol is the main one. Paclitaxel-site is a hydrophobic cleft of the β-tubulin. Several modifications of paclitaxel have also been performed and even new compound families have been developed, as lankacidin group antibiotics [24].
The family of laulimalite / pelorusite bind to the interprotofilament, between two adjacent tubulin dimers, through a crosslinking mechanism [25]. Other compounds that can stabilise microtubules is triazolopyrimidine [26,27] that bind at the same place as vinblastine. Epothilones (maily epothilone B), davunetine and lithium are also stabilizing microtubules.
Other compounds such as toxins destabilize microtubules, inhibiting tubulin growth. Among them, the most known are vinca alkaloids (vinblastine, vincristine). Vinblastine, colchicine and eribulin bind to β-tubulin subunit at the interdimer interfase, but the binding is at different sites [25] Vinblastine binds to the plus-end, perturbing tubuline growth; colchicine substitutes the β-tubulin when growing; eribulin blocks longitudinal tubulin contacts. The pesticide rotenone, inhibitor of complex I from the mitochondrial respiration is another example of destabilizing compound. Other destabilizing compounds are 1-methyl-4-phenylpyrydinium (MPP+), maytansine and 6-hydroxydopamine [28].
Conclusion
The use of different compounds or enzymes affecting microtubule structure can be important cancer and neurodegenerative diseases. Further drugs and proteins must be studied to regulate microtubule dynamics.
References
- Carsten J (2014) The tubulin code: Molecular components, readout mechanisms, and functions. J Cell Biol 206(4): 461-472.
- Mitchison T, Kirschner M (1984) Dynamic instability of microtubule growth. Nature 312(5991): 237-242.
- Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, et al. (2002) HDAC6 is a microtubule-associated deacetylase. Nature 417: 455-458.
- North BJ, Marshall BL, Borra MT, Denu JM, Verdin E (2003) The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell 11(2): 437-444.
- Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, et al. (2010) MEC-17 is an alpha-tubulin acetyltransferase. Nature 467(7312): 218-222.
- Shida T, Cueva JG, Xu Z, Goodman MB, Nachury MV (2010) The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci USA 107(50): 21517-21522.
- Hallak ME, Rodriguez JA, Barra HS, Caputto R (1977) Release of tyrosine from tyrosinated tubulin. Some common factors that affect this process and the assembly of tubulin. FEBS Lett 73(2): 147-150.
- Arce CA, Rodriguez JA, Barra HS, Caputo R (1975) Incorporation of L-tyrosine, L-phenylalanine and L-3,4-dihydroxyphenylalanine as single units into rat brain tubulin. Eur J Biochem 59(1): 145-149.
- Peris L, Wagenbach M, Lafanechère L, Brocard J, Moore AT, et al. (2009) Motor-dependent microtubule disassembly driven by tubulin tyrosination. J Cell Biol 185(7): 1159-1166.
- Sirajuddin M, Rice LM, Vale RD (2014) Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol 16(4): 335-344.
- Mary J, Redeker V, Le Caer JP, Rossier J, Schmitter JM (1996) Posttranslational modifications in the C-terminal tail of axonemal tubulin from sea urchin sperm. J Biol Chem 271: 9928-9933.
- Janke C, Rogowski K, Wloga D, Regnard C, Kajava AV, et al. (2005) Tubulin polyglutamylase enzymes are members of the TTL domain protein family. Science 308: 1758-1762.
- Berezniuk I, Lyons PJ, Sironi JJ, Xiao H, Setou M, et al. (2013) Cytosolic carboxypeptidase 5 removes α- and γ-linked glutamates from tubulin. J Biol Chem 288: 30445-30453.
- Rogowski K, Juge F, van Dijk J, Wloga D, Strub JM, (2009) Evolutionary divergence of enzymatic mechanisms for posttranslational polyglycylation. Cell 137(6): 1076-1087.
- Díaz-Nido J, Serrano L, López-Otín C, Vandekerckhove J, Avila J (1990) Phosphorylation of a neuronal-specific beta-tubulin isotype. J Biol Chem 265(23): 13949-13954.
- Song Y, Kirkpatrick LL, Schilling AB, Helseth DL, Chabot N, et al. (2013) Transglutaminase and polyamination of tubulin: posttranslational modification for stabilizing axonal microtubules. Neuron 78(1): 109-123.
- Caron JM, Vega LR, Fleming J, Bishop R, Solomon F (2001) Single site alpha-tubulin mutation affects astral microtubules and nuclear positioning during anaphase in Saccharomyces cerevisiae: possible role for palmitoylation of alpha-tubulin. Mol Biol Cell 12(9): 2672-2687.
- Shida T, Cueva JG, Xu Z, Goodman MB, Nachury MV (2010) The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci USA 107: 21517-21522.
- Ji S, Kang JG, Park SY, Lee J, Oh YJ, et al. (2011) O-GlcNAcylation of tubulin inhibits its polymerization. Amino Acids 40: 809-818.
- Wong CC L, Xu T, Rai R, Bailey AO, Yates III JR, et al. (2007) Global analysis of posttranslational protein arginylation. PLoS Biol 5: e258.
- Xiao H, El Bissati K, Verdier-Pinard P, Burd B, Zhang H, et al. (2010) Post-translational modifications to Toxoplasma gondii alpha- and beta-tubulins include novel C-terminal methylation. J Proteome Res 9: 359-372.
- Rosas-Acosta G, Russell WK, Deyrieux A, Russell DH, Wilson VG (2005) A universal strategy for proteomic studies of SUMO and other ubiquitinlike modifiers. Mol Cell Proteomics 4(1): 56-72.
- Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4(4): 253-265.
- Ayoub AT, El-Magd RM A, Xiao J, Lewis CW, Tilli TM, et al. (2016) Antitumor activity of lankacidin group antibiotics is due to microtubule stabilization via a paclitaxel-like mechanism. J Med Chem 59(20): 9532-9540.
- Prota AE, Bargsten K, Northcote PT, Marsh M, Altmann KH, et al. (2014) Structural basis of microtubule stabilization by laulimalide and peloruside. A Angew Chem Int Ed 53: 1621-1625.
- Sáez-Calvo G, Sharma A, Balaguer FA, Barasoain I, Rodríguez-Salarichs J, et al. (2017) Triazolopyrimidines are microtubule-stabilizing agents that bind the vinca inhibitor site of tubulin. Cell Chemical Biology 24(6): 737-750.
- Zhang B, Yao Y, CA S, Oukoloff K, James MJ, et al. (2018) A brain-penetrant triazolopyrimidine enhances microtubule-stability, reduces axonal dysfunction and decreases tau pathology in a mouse tauopathy model. Molecular Neurodegeneration 13(1): 59.
- Cartelli D, Cappelletti G (2017) Microtubule destabilization paves the way to Parkinson’s disease. Mol Neurobiol 54: 6762-6774.

 Mini Review
Mini Review