Abstract
We report a case of percutaneous coronary intervention complicated by deformation and dislodgement after successful stent implantation in a patient with multiple risk factors for coronary artery disease and angiographic evidence of three-vessel disease. We performed uneventful angioplasty of proximal portion of left anterior descending artery. Subsequently, coronary angioplasty of left circumflex artery was complicated with stent dislodgement to left main and subsequent onset of hemodynamic instability and refractory cardiac arrest. After stabilization of hemodynamics by means of prompt execution of cardiopulmonary resuscitation maneuvers and institution of Veno-Arterial Extra Corporeal Membrane Oxygenation, crushing the dislodged stent to the vessel wall of the left circumflex artery effectively treated this complication. Veno-Arterial Extra corporeal Membrane Oxygenation was successfully weaned off 24-h after the procedure, the patient recovered completely in the following days and was discharged asymptomatic ten days after the procedure.
Keywords: Stent Complication; Cardiac Arrest; Mechanical Support
Abbreviations: PCI: Percutaneous Coronary Interventions; LAD: Left Anterior Descending; LCX: Left Circumflex; LAD: Left Anterior Descending; RCA: Right Coronary Artery; DES: Drug Eluting Stent; ACT: Activated Clotting Time; ICU: Intensive Care Unit
Introduction
Stent dislodgement is a serious complication during Percutaneous Coronary Interventions (PCI) and potentially responsible for cardiac arrest, a condition requiring a rapid intervention and restoring of coronary and systemic blood flow. Recently, Veno-Arterial ExtraCorporeal Membrane Oxygenation (VA-ECMO) has become readily applicable even in the setting of the catheterization laboratory. In the present paper we report a case of refractory cardiac arrest due to left main thrombosis complicating stent dislodgement, successfully treated with support of VA-ECMO. The patient gave the consent for publication of this anonymous case report.
Clinical Case
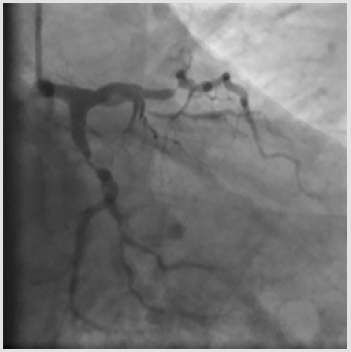
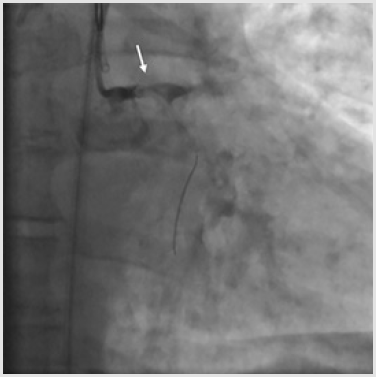
A 62-year-old man with multiple risk factors (dyslipidemia, class 1 obesity, type 2 diabetes mellitus, smoking, obstructive sleepapnea syndrome), stable effort angina and a positive stress test was admitted to our Cardiology Department. Coronary angiography carried out through right radial access revealed sub-occlusive stenoses of the proximal Left Anterior Descending (LAD) artery and of the proximal Left Circumflex (LCx) artery, with a 70% stenosis also on the first Obtuse Marginal Branch (OM1) (Figures 1a & 1b) and a chronic total occlusion of the proximal Right Coronary Artery (RCA) (SYNTAX score 22). The patient refused coronary artery bypass grafting; therefore, it was decided to perform angioplasty on LAD, LCx and OM1 after administration of a 600 mg loading dose of clopidogrel and 6500 IU (70 IU/kg) of unfractioned heparin (the patient was already on aspirin at the time of the procedure).
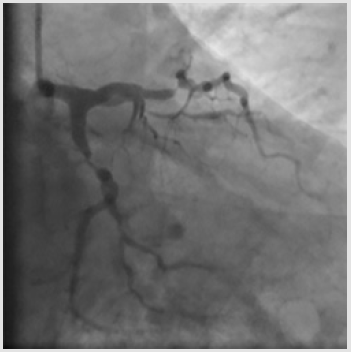
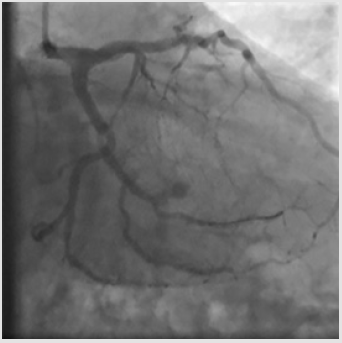
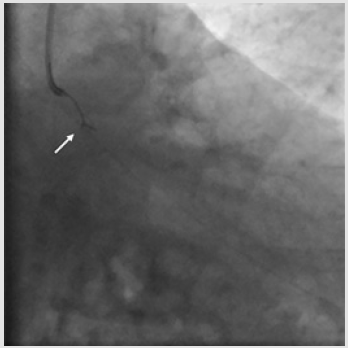
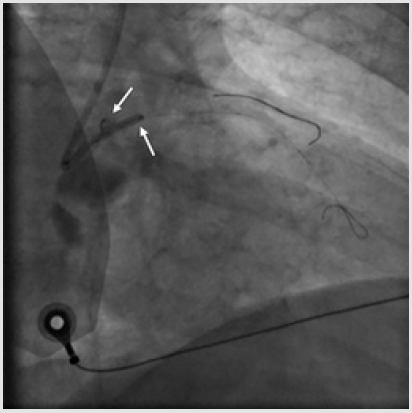
A 6-Fr JL 3.5 guiding catheter was used to engage the Left Main (LM) from the right radial access. Firstly, the LAD stenosis was crossed with a 0.014-inch BMW wire (Hi-Torque Balance Middleweight Wire; Abbott Vascular, Santa Clara, CA, USA). After pre-dilation with a 2.5 x 15 mm semi-compliant balloon (Trek; Abbott Vascular), a 3.5 x 18mm Drug Eluting Stent (DES) (Xience Sierra; Abbott Vascular) was deployed into the LAD up to 15 atm, then post-dilated with a 3.5 x 15 mm non-compliant balloon (NC Trek). Subsequently, the same 0.014-inch BMW wire was removed from the LAD to be positioned in the distal tract of the OM1 and the proximal LCx was pre-dilated with the previous 2.5x15mm semicompliant balloon. Following, a 3.0x15mm DES (Xience Sierra) (Figure 1) was successfully deployed in the proximal OM1, whereas a 3.5 x 15 mm DES (Xience Sierra) was implanted into the proximal LCx at 16 atm for 32 seconds. Post-deployment angiogram showed optimal stent expansion (Figure 2). After complete left coronary revascularization, it was decided to remove the wire; however, during this maneuver, an unexpected resistance was encountered. Subsequent angiography revealed the distal tip of the wire firmly entrapped in the distal edge of the LCx stent and the stent itself appeared deformed (Figure 3). Thus, several unsuccessful attempts of re-crossing the vessel and the damaged stent were carried out using different coronary wires (Intermediate, Abbott Vascular; Whisper, Abbott Vascular; Hi-Torque Pilot 150, Abbott Vascular). At that time, the Activated Clotting Time (ACT) was 310 sec and no additional heparin was administered.
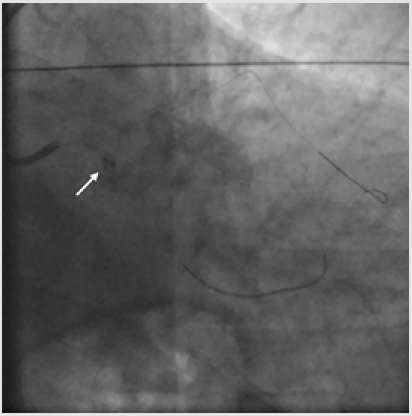
Considering that the wire tip could not be freed, and it proved impossible to advance a second guidewire, it was decided to forcefully pull back in order to retrieve the wire and the deformed stent into the guide catheter. Unfortunately, this attempt resulted in a wire fracture and proximal migration of the stent up to the distal LM. Patient’s hemodynamics was still stable; thus, it was decided to try to capture the dislodged stent by using a 5-mm Amplatz Gooseneck Snare (Medtronic, Minneapolis, MN, U.S.A.). Several attempts were done but without success. After a short while, the patient began to experience ischemic symptoms and hemodynamic instability. Angiography revealed acute thrombosis of the deformed stent, with slow/no forward flow into the LM (Figure 4). Concurrently, the ECG demonstrated initially diffuse ST elevation and QRS complex enlargement, and then ventricular fibrillation. Cardiopulmonary Resuscitation (CPR) and orotracheal intubation maneuvers were performed and 200J shock with an external defibrillator was also administered. Considering that the patient went into refractory cardiac arrest, mechanical support was provided by cardiovascular surgeons that positioned VA-ECMO (Maquet Cardiopulmonary AG, Hirrlingen, Germany) through a femoral access. Notably, during the insertion of VA-ECMO, which took only 11 minutes, manual chest compressions were not interrupted. VA-ECMO support was initiated at a blood flow of 4L/min and quickly enabled to stabilize hemodynamics.
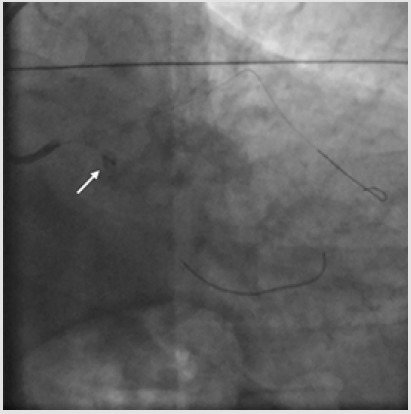
After stabilization was achieved, it was again tried to approach the deformed stent and trap it with the “two-wire technique”, without success. Also, multiple dilations were performed with a 2.5 x 15mm semi-compliant balloon advanced through a wire positioned in the LAD in order to crush the stent against the vessel wall (Figure 5). However, the collapsed stent migrated again towards the ostium of the LAD, with concomitant partial flow restoration and hemodynamic improvement. While another BMW wire was advanced in LCX for vessel protection, the deformed stent moved to the proximal LCx (Figure 6). Thus, dilation with a semicompliant 2.50 x 15mm balloon (Trek) was performed and another DES 3.5 x 18 mm (Resolute Onyx; Medtronic) was used to compress the deformed stent against the LCx wall. A final angiography confirmed that this last stent was successfully implanted alongside the crushed stent; of note, a TIMI 3-flow was achieved on LM, LAD and LCx (Figure 7).
After the procedure, the patient was transferred to the Intensive Care Unit (ICU). After 24 hours from the procedure, ECMO flows were firstly decreased to approximately 1L/min and then removed. Inotropic agents were also gradually weaned off. On the 4th day after the procedure, extubation was performed. On the 5th day after the procedure, vital signs were stable without vasopressors support; therefore the patient was transferred to the general ward. The patient had a full cardiopulmonary and neurological recovery and was discharged 10 days after the procedure on dual antiplatelet therapy, in good conditions, symptom free. The patient remained asymptomatic and free of angina at 3-month follow-up.
Discussion
The incidence of stent dislodgement and loss during PCI has significantly decreased over the last twenty years (0.32-0.02%), probably due to the improved stent design, adjunctive equipment, and stenting techniques [1]. However, considering the increasingly complexity of procedures, this severe complication remains an important issue in interventional cardiology, because potentially associated with intracoronary thrombosis and myocardial ischemia such as in our clinical case [2]. Several procedural mechanisms may contribute to the development of this complication, such as guide catheter compression of stents deployed in an ostial location, compression by post-dilation balloons, inadequate coaxial alignment of the guide catheter and secondary devices use [2,3]. Other anatomical and procedural risky features include extreme coronary angulation, severe tortuosity and/or calcification, diffuse disease, inadequate lesion pre-dilation or debulking, direct stenting, number and length of deployed stents, or previous bypass surgery [2,4]. In our case, a proper pre-dilation was done; however, the unfavorable angulation between the two implanted stents on LCx and OM1 may have contributed to the wire trapping and the stent dislodgement was favored by the wire forced pull-back performed by the operator with the addition of an inadequate coaxiality of the JL guiding catheter towards LCx due to the acute LM-LCx angle.
Finally, new-generation DES are manufactured with thin struts, and undoubtedly are easy to deliver and highly con¬formable. However, this innovative design allows maintaining radial strength, but longitudinal strength may be reduced. Recently, cases of longitudinal compression of stents deployed in ostial position have been published [3].
A deformed/dislodged stent should be recovered when possible. Various retrieval techniques have been described, including the “balloon inflation technique”, use of gooseneck snares, grasping forceps, and stent entrapment with the “twowires” technique or with basket devices [1-7]. However, all of them are time-consuming and if stent retrieval is not possible, particularly in hemodynamically unstable patients, the easier and faster treatment is to use a new stent to crush the dislodged stent against the vessel wall [4]. Although this technique is not widely accepted for LM and proximal LAD dislodgements because of an increased risk for stent thrombosis and restenosis, this approach was recently reported in a published case report, where a dislodged stent was safely crushed to the distal LM/ostial LAD vascular wall in a hemodynamically unstable patient [8]. In case of failure of all percutaneous techniques and according to the dislodgement coronary site (LM or proximal LAD), surgical intervention may represent the last option [5-8].
Certainly, the most important issue during the acute management of this complication is represented by patient’s hemodynamic conditions as well as coronary flow distal to the lost stent. If the patient is unstable with impaired coronary flow, it is crucial to stabilize his/her hemodynamic conditions first and promptly reestablish the coronary flow as quickly as possible [8]. In the present case, initially the patient was asymptomatic, with a preserved distal flow, thus several attempts were firstly carried out to retrieve the dislodged stent. When the condition deteriorated, the priority was to stabilize hemodynamics with VA-ECMO support and then to restore coronary flow with the fastest and most effective way. In fact, survival is low for both in-hospital (15-20%) and outof- hospital (10%) cardiac arrest managed with conventional CPR [9]. Thus, several factors contributed to the complete recovery of our patient. Even though he had several comorbidities, he was relatively young, CPR maneuvers were promptly initiated, VAECMO support was carried out quickly, and the condition causing cardiac arrest was corrected as soon as possible.
Over the past decade, use of temporary Percutaneous Mechanical Circulatory Support (pMCS) devices for cardiac arrest management is increased [10,11]. An ideal pMCS should be readily available, inserted rapidly, associated with low complication rates and cost-effective [10]. The two main pMCS suitable for cardiac arrest management in the catheterization laboratory are VA-ECMO and Impella [10]. The Impella, compared with VA-ECMO, has the advantage of using smaller vascular access and unloads the left ventricle; on the other side, it does not oxygenate the blood, it provides a lower level of hemodynamic support, and does not assist the right ventricle, unless even a right sided Impella is placed [10]. ECMO is usually established by cannulation of a femoral vein and artery circuit and consists of a centrifugal blood pump, an oxygenator and a heparin-coated circuit [12]. VA-ECMO provides both blood oxygenation and bi-ventricular hemodynamic support in patients with cardiopulmonary failure, thereby providing adequate temporary perfusion (non-pulsatile flow) and oxygenation to the organs with subsequent rapid restoration of hemodynamic conditions [12,13]. Notably, the beneficial effects of emergent ECMO implantation in the catheterization laboratory, are more prominent in patients without previous heart failure who suffer refractory cardiac arrest due to a potentially treatable and therefore reversible cause of myocardial ischemia [13]. Of note, in the present case, early ECMO insertion was essential to perform a subsequent successful revascularization. Obviously, since conventional CPR was initiated immediately after onset of ventricular fibrillation, it also played a critical bridging role until ECMO insertion.
Despite the clear benefit of mechanical support, there is a paucity of data regarding emergency use of VA-ECMO in the catheterization laboratory. Few case reports showed that ECMO is becoming increasing available also in this setting and may represent an effective temporizing measure/bridge to the final therapeutic intervention, such as PCI or surgery, that could solve the reversible cause of cardiac arrest (1) [13-15]. In 14 cases of refractory cardiac arrest during PCI or TAVI (Transcatheter Aortic Valve Implantation), ECPR using percutaneous VA-ECMO allowed to restore circulation in all patients, with 50% of survival at hospital discharge [11]. Moreover, surgical ECMO implantation by vessel exposure has been gradually replaced by percutaneous techniques. Indeed, it has been demonstrated that fluoroscopy-guided emergency percutaneous implantation of femoral VA-ECMO can be effectively and safely done by an interventional cardiologist in the catheterization laboratory [14]. Unfortunately, ECMO is still associated with a broad range of complications [11,12]. Also, of note, unlike the other pMCS devices, ECMO increases LV afterload, inducing left ventricular distension [11-15].
Conclusion
Stent deformation and displacement is a rare complication of PCI associated with high mortality. With increased complexity of PCI-treated coronary lesions, interventional cardiologists can deal with this serious and potentially fatal complication. In this article, we have reported the successful treatment of stent dislodgement by crushing technique in a patient with refractory cardiac arrest assisted with VA-ECMO. VA-ECMO provides an effective temporary circulatory support in the catheterization laboratory in case of refractory cardiac arrest and its early initiation can be lifesaving.
Conflict of Interest
The authors have no conflicts of interest to declare.
Established Facts and Novel Insights
Established Facts
a) Procedural complications such as stent dislodgement may
lead to acute myocardial ischemia and cardiac arrest during
PCI.
b) Treatment of these mechanical complications requires a
rapid intervention to restore blood flow.
Novel Insights
a) VA-ECMO can be quickly positioned in case of refractory
cardiac arrest during PCI.
b) VA-ECMO provides an effective temporary circulatory
support in the catheterization laboratory in case of refractory
cardiac arrest and its early initiation can be lifesaving.
References
- Iturbe JM, Abdel Karim AR, Papayannis A, Banerjee S, Brilakis ES, et al. (2012) Frequency, treatment, and consequences of device loss and entrapment in contemporary percutaneous coronary interventions. J. Invasive Cardiol 24(5): 215-221.
- Arnous S, Shakhshir N, Wiper A, Farzin, Paul W, et al. (2015) Incidence and mechanisms of longitudinal stent deformation associated with Biomatrix, Resolute, Element, and Xience stents: Angiographic and case-by-case review of 1,800 PCIs. Catheter. Cardiovasc Interv 86(6): 1002-1011.
- Williams PD, Mamas MA, Morgan KP, Fath Ordoubadi F, Fraser DG, et al. (2012) Longitudinal stent deformation: a retrospective analysis of frequency and mechanisms. EuroIntervention 8(2): 267-74.
- Malik SA, Brilakis ES, Pompili V, Chatzizisis YS (2018) Lost and found: Coronary stent retrieval and review of literature. Catheter Cardiovasc Interv 92(1): 50-53.
- Mehta V, Pandit BN, Yusuf J, Mukhopadhyay S, Trehan V (2014) Retrieval of impacted broken balloon by balloon inflation in guiding catheter. Cardiovasc Interv Ther 29(3): 252-255.
- Kwan TW, Chaudhry M, Huang Y, Pancholy S, Patel T, et al. (2013) Approaches for dislodged stent retrieval during transradial percutaneous coronary interventions. Catheter Cardiovasc Interv 81(6): E245-249.
- Paulus BM, Fischell TA (2010) Retrieval devices and techniques for the extraction of intravascular foreign bodies in the coronary arteries. J Interv Cardiol 23(3): 271-276.
- Stajic Z (2015) Stent dislodgement in the distal left main coronary artery and its successful management with balloon crushing technique. Vojnosanit. Pregl 72(5): 454-457.
- Conrad SA, Rycus PT (2017) Extracorporeal membrane oxygenation for refractory cardiac arrest Ann Card Anaesth 20(Supplement): S4-S10.
- Yadav K, Truong HT (2018) Cardiac Arrest in the Catheterization Laboratory. Curr. Cardiol. Rev 14(2): 115-120.
- Arlt M, Philipp A, Voelkel S, Schmid C, Hilker M, et al. (2012) Early experiences with miniaturized extracorporeal life-support in the catheterization laboratory. Eur J Cardiothorac Surg 42(5): 858-863.
- Keebler ME, Haddad EV, Choi CW, Wigger M, Menachem JN, et al. (2018) Venoarterial Extracorporeal Membrane Oxygenation in Cardiogenic Shock. JACC Heart Fail.
- Lee MS, Pessegueiro A, Tobis J (2008) The role of extracorporeal membrane oxygenation in emergent percutaneous coronary intervention for myocardial infarction complicated by cardiogenic shock and cardiac arrest. J. Invasive Cardiol 20(9): E269-272.
- Goslar T, Knafelj R, Radsel P, Gorjup V, Noc M, et al. (2016) Emergency percutaneous implantation of veno-arterial extracorporeal membrane oxygenation in the catheterisation laboratory. EuroIntervention 12(12): 1465-72.
- Puehler T, Philipp A, Schmid C, Luchner A (2010) Implantation of a new extracorporeal life support system during resuscitation in complicated percutaneous coronary intervention with occlusion of the left main artery. Eur Heart J 31(20): 2499.

 Case Report
Case Report