Abstract
Objective: Phosphoribosylpyrophosphate synthetase-1 (PRPS-1; EC=2.7.6.1) catalyzes the phosphoribosylation of ribose 5-phosphate to 5-phosphoribosyl-1- pyrophosphate, which is the key regulative enzyme of the purine and pyrimidine synthesis. We aimed mainly to define the impact of the microelements (mostly essential metals ions) on the PRPS-1 in the setting of glioblastoma (GB).
Methods: For purification of the PRPS-1 we used the tissue processing, gelfiltration, dialysis, affinity chromatography, RP-HPLC methods. Primer cell culture of the GB cells was maintained by the well-established methods of M. Mattson, 1990. Cells were injected into the cortex of the rat brains.
Results: Adenosine, high concentration of SO42- was acting as the inhibitors for the PRPS-1 (98,7634±1,2450,93,2074±0,0932; p<0.05). The other agents (Co2+, Cu2+, Mg2+) were possessing with the activating abilities on the same enzyme. In the settings of the GB high concentrations of SO42- were diminishing the activity of PRPS-1 in the experimental GB settings in comparison with the control. It was notable elevation of the adenosine deaminase as well as xanthine oxidoreductase activities in the same groups in comparison with the control animals.
Conclusion: The inhibitors of PRPS-1 might be useful in the setting of the cancer prevention, particularly for GB.
Keywords: Phosphoribosylpyrophosphate Synthetase-1; Microelements; Metal Ions; Adenoisne Deaminase; Xanthine Oxidoreductase; Glioblastoma
Abbreviations: BSA: Bovine Serum Albumin, GB: Glioblastoma, OPT: Orotate Phosphoribolsyl- Transferase, XDH: Xanthine Dehydrogenase
Introduction
Phosphoribosylpyrophosphate synthetase-1 [PRPS-1; EC=2.7.6.1) catalyzes the phosphoribosylation of ribose 5-phosphate to 5-phosphoribosyl-1-pyrophosphate, which is necessary for the salvage pathways of purine and pyrimidine, pyridine nucleotide cofactors NAD and NADP, the amino acids histidine and tryptophan biosynthesis [1,2]. It is regulative enzyme, responsible for the synthesis of purine and pyrimidine. Regulation of this particular enzyme might be dependent on the effectors, including metal ions. Generally, metal ions are vital for functionality (metallo-enzyme activity, protein stabilization etc.) and maintenance of nervous tissue. The overload of copper lead to neurodegeneration in Menkes and Wilson’s disease, increased brain aging, dwindle cognitive and epileptic seizures due to altered brain zinc homeostasis. Presence of dyshomeostasis of essential and nonessential metals is considered vital in sporadic neurodegeneration. Increased levels of iron and copper in tissue are directly related to increased inflammatory markers and oxidative stress in affected brains. The rich metal (zinc, iron, copper) concentration in degenerated protein aggregates and plaques demonstrates the link between metals and neurodegenerative pathologies. Changes in aggregation properties (a-synuclein and amyloid-b) lead to complex protein formation owing to the presence of aluminium, copper and iron [3].
Super activity of PRPS is an inherited X-chromosome-linked
disorder [4] and the excessive enzymatic activity is associated with uric acid overproduction, gout and neurodevelopmental
abnormalities [5-8]. Thus, in case of the genetic pathologies the
regulation of PRPS-1 is essential. Three classes of PRSPs have
been reported which are divided based on their dependence on
phosphate ions for activity, their allosteric regulation mechanism
and their diphosphoryl donor specificity [9-12]. Most PRSPs belong
to the class I, which require Mg2+ and phosphate for enzymatic
activity, but can be inhibited allosterically by ADP and possibly
other nucleotides [13-20]. Class II PRSs are found specifically
in plants which are not dependent on phosphate for activity and
lack an allosteric site for ADP [9-10]. Recently, a novel class III PRS
has been identified from Methanocaldococcus jannaschii which is
activated by phosphate and uses ATP and dATP as a diphosphoryl
donor, but also lacks an allosteric site for ADP [12]. HPRPSs
(human PRP synthetases) have three isoforms that share very high
sequence identity (95.0% between hPRPS1 and hPRPS2; 94.3%
between hPRPS1 and hPRPS3; and 91.2% between hPRPS2 and
hPRPS3 respectively) [21-24].
Enzyme requires phosphate for activation and uses Mg2+
[14,25-27]. The crystallization of hPRPS1 has recently been reported
[27]. Interestingly, in addition to binding at the R5P binding site
and the allosteric site defined previously in bsPRS, an extra SO42−
ion is found to bind at a new allosteric site at the dimer interface.
Structural and biochemical data together reveal new insights into
the allosteric regulatory mechanism of hPRPS1 and possibly other
eukaryotic PRPSs (except for class II plant PRSs) [28]. In our previous
publications we have proposed that activation of PRPS-1 in the
brain might trigger the increase in the rate of the purine and pyrimidine
biosynthesis and stimulate the regenerative processes after
experimental stroke in rats [29]. The treatment of the animals with
the phosphates after experimental stroke reflecting conditions, we
have noticed the elevation of the Ki-67 positive neurons in the brain
as well as the decrease in the BBB (blood brain barrier) damage
process and Evans Blue extravasation [29].
In our current study we were aiming to define non-organic
and organic compounds, which are influencing on the activity of
the PRPS-1. In addition, we developed the methods for Orotate
Phosphoribolsyl-Transferase (OPT) and PRPS-1 purification and
creation of the kit (Figure 1), measuring the PRPS-1 activity in blood
as well as in tissues. We established the Glioblastoma (GB) model
and evaluated the inhibitor of PRPS-1 – high and low concentration
of SO42− ions. Along with the PRPS-1 we measured the adenosine
deaminase -2 (ADA-2) as well as Xanthine Oxidoreductase (XOR)
activities as the potential markers of GB.
Material and Methods
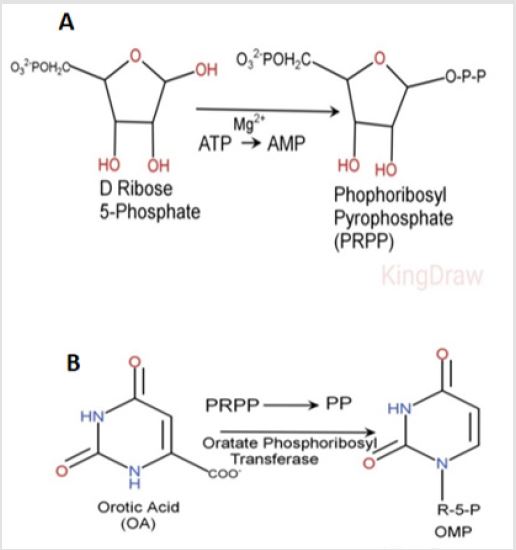
The all reagents were purchased from Sigma/Aldrich or/and Santa Cruz Biotechnology, Inc. We have created the kit for the determination of the PRPS-1 activity. The entire idea of the leaded reactions for the final measurement of the enzyme activity is presented on the (Figure 1).
Figure 1: The synthesis of PRPS from D-Ribose 5-Phosphate by the PRPS-1 and the following formation of the Ribose 5-Phospahateand PP in the presence of OPT.
Purification of Orotate Phosphoribolsyl-Transferase (OPT)
The brain tissue, predominantly cortical part of the human
brain was obtained from the carcasses of the death bodies. The
tissue was desiccated in accordance to the permission of the
relatives to use the tissues for the bench-top scientific experiments.
The experimental procedures with the human tissues was
approved by the Ethical Committee of the H Buniatian Institute
of Biochemistry of the National Academy of Science of Armenia
(Reference Letter N 2; Active International Registrations’ Numbers:
IRB0001621, IORG 0009782). Around 200-250g of the tissue was
washed with the basic buffer, containing 10mM Tris buffer, I mM
EDTA, 1 mM trypsine inhibitor obtained from the soy beans and
0.001M KNaC4H4O6x4H2O (pH=7.8). After all, we have performed
glass-glass homogenization in the cold conditions ( 40C) with the
basic buffer, containing sucrose. The homogenate was centrifuged
at 14,000g for 30 minutes at ْ 4 C and the resultant supernatant
fraction was used as the starting material.
The proteins were precipitated with 50% ammonium sulfate.
After centrifugation at g=14000 the protein fraction was placed into
the dialysis bags against 0.1M PBS. The pooled fractions were than
fractionated with the cold acetone (-200 C). Cold acetone was added
with vigorous stirring to the enzyme fraction until an acetone/
homogenate would reach to the 43:57 ratio. The preparation
was immediately centrifuged at 5000g for 10 minutes at ْ 40 C. The
resultant supernatant was discarded and the pellet resuspended
in 50ml basic buffer. The ready fraction was applied to 300ml of
DEAE-Sephacel preequilibrated with basic buffer. After application,
the gel was washed with three 200ml aliquots of basic buffer and
the enzyme was then eluted by washing the column with 100ml
aliquots of 0.05-0.2M NaCl basic buffer. The fractions were collected
and assayed for the enzyme activity. The protein fractions were
placed to dialysis against 0.1MPBS. The fractions were applied to a
35x 2.5sm DEAE-Sephacel column preequilibrated with buffer.
Elution was performed with 0.05-0.2M NaCl gradient in basic
buffer. The fraction were collected and assayed for the enzyme
activity. Further, the fractions were concentrated with 50%
ammonium sulfate and placed for dialysis against 0.1M PBS. Affinity
chromatography was performed by the utility of the modified
Cibacron Blue gel (Santa Cruz Biotechnology, USA). Cibacron Blue
contains amino-groups on the surface. Aldehyde might bind amino
groups of orotic acid and the amino-groups of Cibacron Blue with
each other. It was used 0.001% orotic acid (Santa Cruz Biotechnoloy,
USA) for the binding purpose. Due to partial structural modifications
orotic acid doesn’t bind permanently with OPT and in conditions of
application of the high orotic acid solution (elution with the 0.01%)
the bound OPT might be washed out. The effectively of the binding
was determined by the scanning of the orotic acid and washed out
eluent after addition of the same quantity of orotic acid on the gel
vs pure orotic acid solution with the same amount of the acid. Final
washed out eluent was containing negligible amount of orotic acid,
which proved the fact of the binding of the applied orotic acid with
the Cibacron Blue by the glutaraldehyde (50%, Sigma-Aldrich).
Purity of the obtained enzymes was detected by the RP-HPLC.
Purification of the PRPS-1
The enzyme was purified from the human brain cortex. The routine purification procedures were the same as for the OPT. Semi-affinity chromatography was performed by the utility of the Cibacron Blue gel (Santa Cruz Biotechnology, USA). The elution was performed by the 44% NAD+ (Sigma-Aldrich). Purities of the obtained enzymes were detected by the RP-HPLC.
Measurement of PRPS-1 activity
Purified PRPS-1 activity was determined in the presence of purified OPT, orotate (0.2mM), ATP (0.3mM), Ribose-5-phosphate (4mM), magnesium ions, 1M Tris buffer (pH=7.7), 36.6C◦ after 60 minutes incubation and detection at the λ=730 nm by the utility of the Cary 60 spectrophotometer (Agilent, USA). Coloring solution was containing the concentrated sulfuric acid, 4% ascorbic acid, 0.3% anthimony potassium tartrate and sodium molybdate (4.5%). The absorption was detected by the Cary 60 Spectrophotometer (Agilent, USA) at 730nm wavelength. For the calculation of the specific activity of the enzymes it was applied the Bradford method, determining the quantity of the proteins in solution.
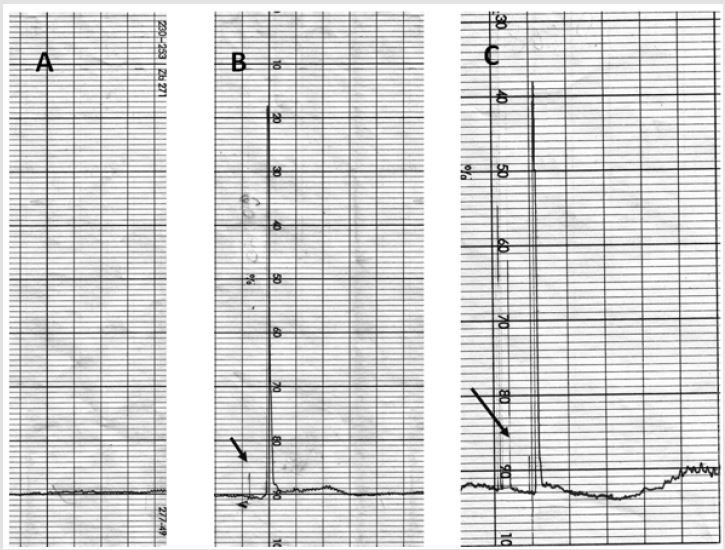
RP-HPLC: For the final detection of the purity of the obtained enzymes it was used Shimadzu LC -20 chromatograph, supplied with UV-Vis detector SM 5000 and the C18 RP-colums of the Avex, Waters, Symmetry companies. The speed of the elution was 2ml/min. As the eluents there was used degased water as well as acetonitrile (Sigma-Aldrich) in the ratio of 20:80. The enzymes absorption was detected at 280nm. The analyses were performed in 10 minutes (Suppl. Figure 1).
Supplementary Figure 1: RP-HPLC evaluation of purified PRPS-1 and OPT.
A. Background of the eluent.
B. PRPS-1.
C. OPT. The arrows are pointing the draft for the starting point of the analyses.
Methyl Green
Methyl green (2% (w/v) in 0.1M NaOAc, pH 4.2 was prepared. It was mixed 918ml of 0.1N acetic acid with 331mL of 0.1M NaOAc and adjust pH to 4.2 with NaOH. The final solution was containing 25gm of methyl green dye. The solution was filtered through Whatman #2 filter paper. After application of the solution on the frozen slices for 5-10 minutes, they were washed and visualized by the light microscopy (Trinocular; Boeco, Germany, 1800 magnification) [30].
Cell Culturing
GB tissue was withdrawn and placed in Neurobasal medium (NB, prenatal, Gibco Life Technologies) containing 0.05% Bovine Serum Albumin (BSA). The tissue was isolated and incubated at 37⁰C for 20 min in NB containing 0.05% BSA, 0.15% trypsine. Tissue was resuspended in fresh NB and mechanically disintegrated using a Pasteur pipette. The supernatant was discarded and the cell suspension was resuspended in NB medium containing 1% BSA. This procedure was repeated 3 times. Human brain cells were collected (1,000rpm, 10min), washed and cultured at 37⁰C, 5% CO2 in 35mm Petri dishes pre-coated with poly-L-lysin (Sigma) containing 0.09% Na2HPO4, 1% glucose, 0.4% KCl, 0.06% KH2PO4, 0.4% MgSO4 x 7H2O and 0.001% gentamicin sulfate. A day later the medium was replaced by NB containing 2% B27-supplement (Gibco) and the cells’ number was calculated on 1st and 3rd days [31].
Animal Model of GB
For the entire n vivo study it was used 70 animals. The all experimental procedures were approved by Ethical Committee of the Institute of Biochemistry, National Academy of Science of Armenia. The animals were anesthetized by administration of pentobarbital 10 mg/ 100 mg of weigh kg, injected I.P. using a hypodermic needle. During surgery cranial and rectal temperature continuously were controlled as these parameters reflecting the normal animal physiological state. The body as well as cranial temperatures was sustained for 36.6 C The hair layer of the white laboratory rats head was removed and the skin before the incision was cleaned with ethanol. The rats were placed in a stereotaxic frame (Poland), and a specially fabricated 27-gauge stainless steel cannula with 30° bevel was introduced after limited craniotomy through a burr hole into the midcerebral cortex.
Cells were in the amount of 8000 injected after limited craniotomy into the brain parenchyma by the following coordinates from bregma: 2mm lateral to midline, 4mm anterior to the coronal suture, and 2mm below the surface of the skull. The volume of injected solution was equal to the 10ul. After infusion, the injection syringe cannula was left in the brain parenchyma for a minute and then removed slowly. After all, the burr hole was covered with the bone wax and the incision was closed. Animals were placed into the cages with the free access for water and food. The hole in the scalp was covered by the paraform based covering gel. Twenty days later animals were decapitated after intacardiac perfusion with Saline. During 29days the animals were injected with the immune suppressants.
Statistics
In our calculations we have used t-test (student) for pair comparison as well as ONE-WAYANOVA for the calculation of the significance of the comparable all groups. The results were considered statistically significant when p was lower or equal to 0.05. In some calculations we used t-student test. There were used sigma Plot 10 and Sigma Stat 10 programs. For the creation of the structural formulas there was used the King Draw program.
Results
The Impact of The Activating Metal Ions on the PRPS-1.
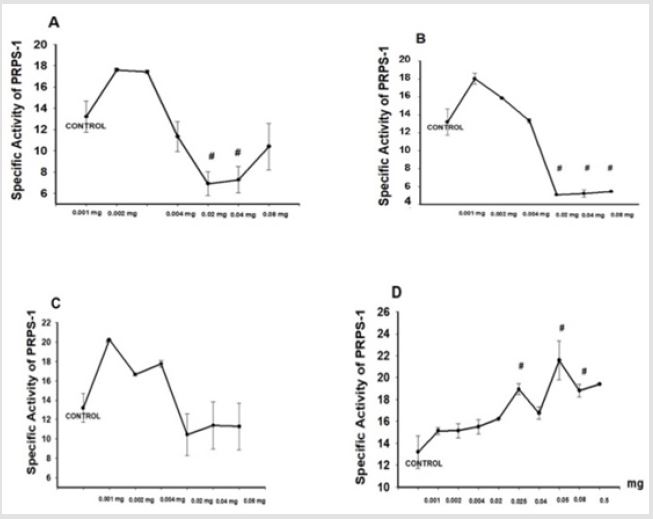
Mentioned all statistically significances were between the groups and control (#). The impact of the ions on the activity of PRPS-1 was estimated by the addition of different concentrations of Co2+, Cu2+, Mg2+ (Figure 2). Addition of the 0.001, 0.002, 0.004 mg of the Co2+ into the mixture was mostly increasing the activity of the PRPS-1 activity in comparison with the control - 13,1934±1,4805 vs 17,6013±0,1465, 17,4197±0,1523, 11,3319±1,4296. Higher 0.02, 0.04, 0.08mg concentrations decreased the activity of the PRPS-1 until 6,9079±1,1303 (p#<0.05), 7,2726±1,2437 (p#<0.05), 10,3969±2,1931. Ions of Cu2+ were decreasing the activity of PRPS-1 in dose dependent manner, however, the activity was higher in the presence of the ions in comparison with the control - 13,1934±1,4805 vs 18,0173±0,638, 15,8523±0,0439, 13,3211±0,2607 and high doses were diminishing that activity until 5,0741±0,0184 (p#<0.05), 5,2080±0,4125 (p#<0.05), 5,4376±0,0677 (p#<0.05). Mg2+ ions were elevating the PRPS-1 activity in low concentrations in comparison with the control 13,1934±1,4805 vs 20,2234±0,0732, 16,6580±0,1523, 17,7478±0,3457 (0.02, 0.04, 0.08 mg, respectfully) and elevating in high concentrations 10,4313±2,1543, 11,3982±2,4453, 11,2791±2,3981 for 0.001, 0.002, 0.004 mg, respectfully.
Influence of the Fosfomycin on PRPS-1 activity.
Increase of fosfomycin concentration (0.001, 0.002, 0.004 , 0.02, 0.025, 0.04, 0.05, 0.08 ) was mostly elevating the activity of PRPS-1 in comparison with the control - 13,1934±1,4805 vs 15,1302±0,3240, 15,1471±0,6372,15,5051±0,6372,16,2303±0,04 78, 18,9460±0,4775, 16,7487±0,5601, 21,5710±1,7959 (p#<0.05), 18,8024±0,5771 (p#<0.05), 19,4001±0,0879 (p<0.04), (Figures 2A- 2D).
Figure 2: Impact of the effectors on the activity PRPS-1. Final 1 ml solution was containing OPRT, PRPS1 orotate, ribose-5 phosphate, tris buffer (pH=7.4), as well as ATP. Incubation was performed during 60 minutes at 36.6 C. Addition of ascorbic acid/sulfuric acid/ammonium molybdate solution/antimony potassium tartrate promoted formation of the stable blue color during 10 minutes. The absorbance of the solution was measured at 730 nm (Cary 60, Agilent, USA). A. Influence of the Co2+ on the activity PRPS-1. B. Influence of the Cu2+on the activity PRPS-1. C. Influence of the Mg2+ ions on the activity PRPS-1. D Influence of the fosfomycin on the activity PRPS-1. It was applied student t-test and p#<0.05.
Impact of the Inhibitors on PRPS-1 activity.
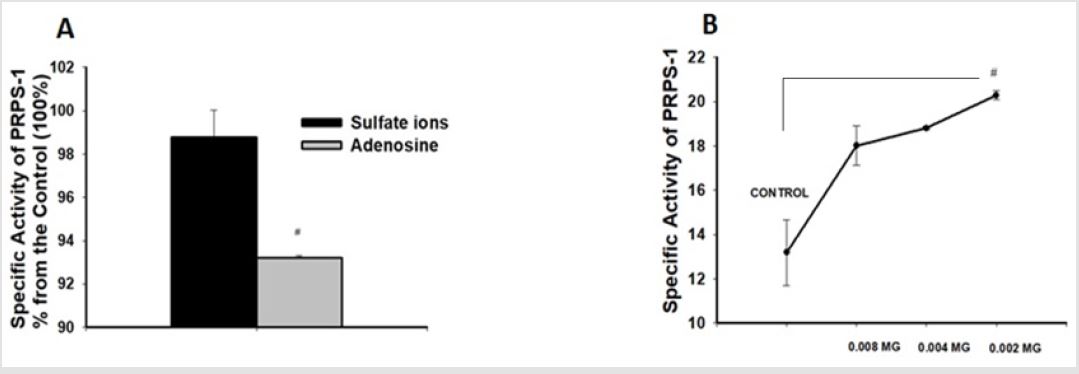
Sulfuric acid ions in the quantity of 0.04mg as well as adenosine were decreasing the activity of PRPS-1 activity in comparison with the 100% control 98,7634±1,2450, 93,2074±0,0932 (p<0.05), (Figure 3).
Low Concentration of SO42- influence on PRPS-1 activity.
Low concentration of SO42- were elevating PRPS-1 in dose dependent manner in comparison with control-13,1934±1,4805 vs18,0144±0,8935, 18,7937±0,0674, 20,2790±0,2051 (p<0.05), (Figures 3A-3B).
Figure 3: Influence of the sulfate ions as well we adenosine on the activity of the PRPS-1.
ions into the reactive mixture. Adenosine was added in the same quantity. The inhibition percentile was calculated from the
control specific activity, which was accounted as the 100%. Measurements were performed by the Cary 60 spectrophotometer
at 730 nm wavelength. The difference between control group and adenosine were statistically significant, p#<0.05, t-student
test.
B. Influence of the low concentrations of the sulfate ions on the activity of PRPS-1. It was applied ONE-WAY-ANOVA for
calculation of the significance and p#<0.04.
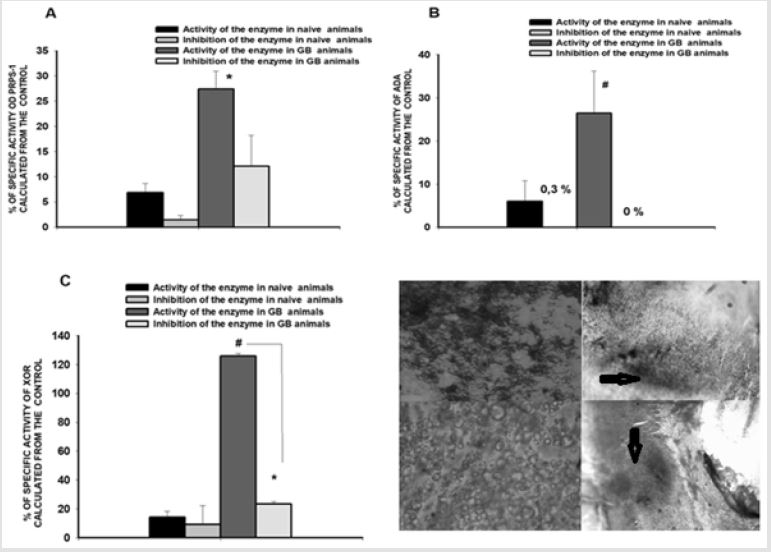
Measurement of the PRPS-1, ADA-2 as well as XOR activities in the settings of experimental GB.
Figure 4: Determination of PRPS-1, ADA and XOR activities in serum of scheme vs GB rats.
A. PRPS-1 activity in control (first two colums) vs GB rats (3th, 4th columns). B. ADA activity in control and GB rats. C. XOR activity in the control and GB rats. D. D1, D2. Glioblastoma cells culture: D2-light microscopy with 10x objective and D1 is the Methyl Green same culture. D3, D4. Methyle Green stained slices of the brain of the animals suffering from glioblastoma (n=6). It is clearly seen the hippocampus area on the figure D (4X objective, light microscopy with the 1800 magnifications (Boeco, Germany) and accumulation of the chromatin on the figure D3 with 10X objective magnification. Arrows are indicating the areas of tissue damage. The statistical significance was noticed between naïve animals and GB aniamls comparison groups without the inhibitors for the all 3 enzymes (p#<0.05, student t-test, p#<0.03 for PRPS-1 activity evaluation, student t-test) and inside the GB group for XOR activity measurement (p*<0.05, student t-test).
PRPS-1 activity (% from the control) was in naïve animals equal to 6,8737±1,8079 (without the inhibitor); after the addition of the inhibitor it was diminished until 1,4136±0,9500 (with the inhibitor inhibitor) vs glioblastoma suffering animals 27,3567±3,5750 (without the inhibitor); 12,0703±6,1529 (with the inhibitor); ADA activities for the same comparison groups were 5,9862±4,7916; 0,3000±0,0000vs 26,4606±9,7808; 0,0000±0,0000, whereas the XOR activity was 14,1968±4,2366; 9,1332±13,2417 vs 125,8324±1,8673; 23,4042±1,7613. The statistical significance was noticed between naïve animals and GB animals comparison groups without the inhibitors for the all 3 enzymes (p#<0.05, student t-test) and inside the GB group for XOR activity measurement (p*<0.05, student t-test), (Figures 4A-4D). As the inhibitors for PRPS-1 were serving high concentration of SO42- ions, for ADA – desoxyadenosine and for XOR- allopurinol.
Discussion and Conclusions
Mg2+ forms a complex with ATP (Mg–ATP) to act as the actual
substrate of PRPS-1 although other divalent cations, such as Mn2+,
Ni+, Co2+ or Cd2+ can serve as the substitutes for Mg2+ with
relatively lower activity [13-15,19,20,32]. Phosphate has multiple
effects on the activity and structure of the enzyme. It usually acts
as an activator for the activity of bacterial and mammalian PRSPs
although SO42− can mimic the effect of phosphate at approx. 10-fold
higher concentrations [11,13,16,19,33]. However, phosphate or
SO42− has to compete with the inhibitor ADP at the same allosteric
site for their function. That is the reason why high concentration
of SO42− in our experiments were suppressing the PRPS-1 activity
whereas the low concentrations had the opposite impact.
Mostly the all ions in low concentrations as well as fosfomycin
had the activating PRPS-1 character and might be used as the
potential compounds to trigger the biosynthesis of the purines and
pyrimidine, which might serve as the basis for the stimulation of
the regenerative processes. In the settings of GB it was notable the
elevation of the PRPS-1, ADA as well as XOR activities. We propose,
PRPS-1 activation is evidencing about the cells pathological
proliferation in the settings of experimental GB. Regulation of
PRPS-1 activity by the microelements might control the growth and
proliferation of atypical glial cells. Adenosine deaminase [34] is a
key enzyme in metabolism of purines. It catalyzes the irreversible
hydrolysis of adenosine into inosine and ammonia. It has a certain
role in maintaining immune competence. The consequence of some
point mutations in active catalytic center of ADА resembles as a
phenotypical representation of SCID. It was shown that in humans
ADA activity occurs mainly in two distinct isoenzymes and they
are referred to as adenosine deaminase 1 (ADA*1) and adenosine
deaminase 2 (ADA*2). ADA*1 exists in two major forms: a monomer
of molecular weight 33,000 (small form) and a dimer-combining
protein complex with a total molecular weight of 280,000 (large
form), earlier called ADA-S and ADA-L.
This complex has no signifcant influence on its catalytic activity.
ADA*2 exists only as a monomer with molecular weight of 100,000
[35-36]. The main immunological function of ADA is regulation of
T, B- cells differentiation as well as B-cells proliferation [35]. At
sites of inflammation and tumor growth, the local concentration
of extracellular adenosine rapidly increases and plays a role in
controlling the immune responses of nearby cells. Adenosine
deaminases ADA1 and ADA2 (ADAs) decrease the level of adenosine
by converting it to inosine, which serves as a negative feedback
mechanism. Mutations in the genes encoding ADAs lead to impaired
immune function, which suggests a crucial role for ADAs in immune
system regulation [37]. We suggest, ADA activity elevation in GB
settings is a marker of the activation of the immune system. Group
of scientists from Italy came to the conclusion, tumors with high
levels of Xanthine Dehydrogenase (XDH) mRNA are characterized
by higher expression of several genes encoding pro-inflammatory
and immune cytokines, and increased levels of tumor infiltration
with immune cells. The group also underlined the existence of
great differences in uricogenesis between different types of human
tumors making XDH as the molecular biomarkers of the cancer and
cancer types [38].
Similar association between the high uricemia with the metabolic
syndrome, diabetes, and cancer was suggested by the numerous
other authors [39]. In our experiments we noticed the elevation
of XOR activity in GB settings in comparison with the scheme, naïve
rats. Thus, we suggest, measurement of 3 above mentioned enzymes
activities for the characterization of experimental GB might
be informative and serves as the diagnostic tool.
Acknowledgment
The work was made possible because of ANSEF biochem-4414 award (PI: Kristine Danielyan). The work was supported also by the basic funding from National Academy of Science of Armenia as well as the grant from Science Committee of Ministry of Education and Science of Armenia (PI: Samvel G. Chailyan-18T‐1F089).
References
- Hove Jensen B (1988) Mutation in the phosphoribosylpyrophosphate synthetase gene (prs) that results in simultaneous requirements for purine and pyrimidine nucleosides, nicotinamide nucleotide, histidine and tryptophan in Escherichia coli. J Bacteriol 170: 1148-11
- Hove Jensen B (1989) Phosphoribosylpyrophosphate (PRPP)-less mutants of Escherichia coli. Mol Microbiol 3: 1487-14
- Ramachandra Rao S (2006) Waste Management Series. Chapter 11 - Recycling of Water and Reagents.
- Yen RC, Adams WB, Lazar C, Becker MA (1978) Evidence for X-linkage of human phosphoribosylpyrophosphate synthetase. Proc Natl Acad Sci USA 75(1): 482-485
- Zoref E, De Vries A, Sperling O (1975) Mutant feedback-resistant phosphoribosylpyrophosphate synthetase associated with purine overproduction and gout. Phosphoribosylpyrophosphate and purine metabolism in cultured fibroblasts. J Clin Invest 56(6): 1093-103
- Becker MA, Losman MJ, Kim M (1987) Mechanisms of accelerated purine nucleotide synthesis in human fibroblasts with superactive phosphoribosylpyrophosphate synthetases. J Biol Chem 262(12): 5596-5
- Roessler BJ, Nosal JM, Smith PR, Heidler SA, Palella TD, et al. (1993) Human X-linked phosphoribosylpyrophosphate synthetase superactivity is associated with distinct point mutations in the PRPS1 gene. J Biol Chem 268(35): 26476-264
- Becker MA, Smith PR, Taylor W, Mustafi R, Switzer RL (1995) The genetic and functional basis of purine nucleotide feedback-resistant phosphoribosyl-pyrophosphate synthetase superactivity. J Clin Invest 96: 2133-21
- Krath BN, Eriksen TA, Poulsen TS, Hove Jensen B (1999) Cloning and sequencing of cDNAs specifying a novel class of phosphoribosyl diphosphate synthase in Arabidopsis thaliana. Biochim Biophys Acta 1430: 403-408.
- Krath BN, Hove Jensen B (2001) Class II recombinant phosphoribosyl diphosphate synthase from spinach. Phosphate independence and diphosphoryl donor specificity. J Biol Chem 276: 17851-1785
- Eriksen TA, Kadziola A, Bentsen AK, Harlow KW, Larsen S (2000) Structural basis for the function of Bacillus subtilis phosphoribosyl-pyrophosphate synthetase. Nat Struct Biol 7(4): 303-30
- Kadziola A, Jepsen CH, Johansson E, Mc Guire J, Larsen S, (2005) Novel class III phosphoribosyl diphosphate synthase structure and properties of the tetrameric, phosphate-activated, non-allosterically inhibited enzyme from Methanocaldococcus jannaschii. J Mol Biol 354: 815-8
- Switzer RL (1969) Regulation and mechanism of phosphoribosylpyrophosphate synthetase. I. Purification and properties of the enzyme from Salmonella typhimurium. J Biol Chem 244(11): 2854-28
- Fox IH, Kelley WN (1971) Human phosphoribosylpyrophosphate synthetase. Distribution, purification, and properties. J Biol Chem 246: 5739-57
- Switzer RL, Sogin DC (1973) Regulation and mechanism of phosphoribosylpyrophosphate synthetase. V. Inhibition by end products and regulation by adenosine diphosphate. J Biol Chem 248: 1063-10
- Roth DG, Shelton E, Deuel TF (1974) Purification and properties of phosphoribosyl pyrophosphate synthetase from rat liver. J Biol Chem 249: 291-29
- Roth DG, Deuel TF (1974) Stability and regulation of phosphoribosyl pyrophosphate synthetase from rat liver. J Biol Chem 249: 297-301.
- Gibson KJ, Schubert KR, Switzer RL (1982) Binding of the substrates and the allosteric inhibitor adenosine 5′-diphosphate to phosphoribosylpyrophosphate synthetase from Salmonella typhimurium. J Biol Chem 257: 2391-239
- Hove Jensen B, Harlow KW, King CJ, Switzer RL (1986) Phosphoribosylpyrophosphate synthetase of Escherichia coli. Properties of the purified enzyme and primary structure of the prs gene. J Biol Chem 261: 6765-67
- Arnvig K, Hove Jensen B, Switzer RL (1990) Purification and properties of phosphoribosyldiphosphate synthetase from Bacillus subtilis. Eur J Biochem 192: 195-200.
- Iizasa T, Taira M, Shimada H, Ishijima S, Tatibana M (1989) Molecular cloning and sequencing of human cDNA for phosphoribosylpyrophosphate synthetase subunit II. FEBS Lett 244: 47-50.
- Sonoda T, Taira M, Ishijima S, Ishizuka T, Iizasa T (1991) Complete nucleotide sequence of human phosphoribosylpyrophosphate synthetase subunit I (PRSI) cDNA and a comparison with human and rat PRPS gene families. J Biochem 109: 361-36
- Taira M, Iizasa T, Shimada H, Kudoh J, Shimizu N (1990) human testis-specific mRNA for phosphoribosylpyrophosphate synthetase that initiates from a non-AUG codon. J Biol Chem 265: 16491-1649
- Nosal JM, Switzer RL, Becker MA (19930) Overexpression, purification, and characterization of recombinant human 5-phosphoribosyl-pyrophosphate synthetase isozymes I and II. J Biol Chem 268: 10168-101
- Fox IH, Kelley WN (1973) Human phosphoribosylpyrophosphate (PP-ribose-P) synthetase: properties and regulation. Adv Exp Med Biol 41: 79-86.
- Fox IH, Kelley WN (1972) Human phosphoribosylpyrophosphate synthetase. Kinetic mechanism and end product inhibition. J Biol Chem 247: 2126-21
- Tang W, L iX, Zhu Z, Tong S, Li X, et al. (2006) Expression, purification, crystallization and preliminary X-ray diffraction analysis of human phosphoribosyl pyrophosphate synthetase 1 (PRS1). Acta Cryst F62: 432-43
- Li S, Lu Y, Peng B, Ding J (2007) Crystal structure of human phosphoribosylpyrophosphate synthetase 1 reveals a novel allosteric site. Biochem J 401(1): 39-47.
- Danielyan KE, Vardanyan, R, Paronyan ZK, Barkhudaryants IM, Bisharyan MS (2018) PRPS-1 is a regulative for neuroprotection and cells regenerative proliferation. Biomolecules and Biochemistry 2(1): 6-10.
- Sullivan Brown J, Bisher ME, Burdine RD (2011) Embedding, Serial Sectioning and Staining of Zebrafish Embryos Using JB-4™ Nat Protoc 6(1): 46-55.
- Mattson MP, Ruchlik B (1992) Cell culture of cryopreserved human fetal cerebral cortical and hippocampal neurons: neuronal development and resposes to trophic factors. Brain Research 552: 2004-2014.
- Willemoes M (1997) Hove-Jensen B. Binding of divalent magnesium by Escherichia coli phosphoribosyl diphosphate synthetase. Biochemistry 36: 5078-50
- Meyer LJ, Becker MA (1977) Human erythrocyte phosphoribosylpyrophosphate synthetase. Dependence of activity on state of subunit association. J Biol Chem 252: 3919-39
- Ficara F, Herna D, Mocchetti C, Carballido Perrig N, Sara Deola GA, et al. (2004) IL-3 or IL-7 Increases ex Vivo Gene Transfer Efficiency in ADA-SCID BM CD34+ Cells while Maintaining in Vivo Lymphoid Potential. Mol Therapy 10(6):
- Cristalli G, Constanzi S, Lambertucci C, Lupidi G, Vittori S, et al. (2001) Adenosine deaminase: functional implications and different classes of inhibitors. Med Res Rev 21(2): 105-1
- Cowan MJ, Brady R, Widder KJ (1986) Elevated erythrocyte adenosine deaminase activity in patients with acquired immunodeficiency syndrome. PNAS 83:
- Kaljas Y, Liu C, Skaldin M, Wu C, Zhou Q, et al. (2017) Human adenosine deaminases ADA1 and ADA2 bind to different subsets of immune cells. Cell Mol Life Sci 74(3): 555-5
- Saidak Z, Louandre C, Dahmani S, Sauzay C, Guedda S, et al. (2018) A pan-cancer study of the transcriptional regulation of uricogenesis in human tumours: pathological and pharmacological correlates. Biosci Rep 38(5).
- Garcia Gil M, Camici M, Allegrini S, Pesi R, Petrotto E (2018) Emerging Role of Purine Metabolizing Enzymes in Brain Function and Tumors. Int J Mol Sci 1.

 Research Article
Research Article