Abstract
Close contact and poor hygiene are major factors for passing around the communicable virus and infectious bacterial. Hand sanitizer is a popular product which can be used for fighting against the hazardous bacterial, thus providing the necessary health protection. It is especially beneficial for the cases when sinks are unavailable, or when sensitive skin would readily suffer from the fissures caused by frequent handwashing. Based on its active ingredients, hand sanitizer can be classified into alcohol-based and alcoholfree products. Of which, alcohol-free sanitizer is under rapid development due to its less skin irritation and persistent antibacterial effect. Nanocellulose, as a leading-edge cellulose derivative, its unique application as continuous phase for the sanitizer gel, was particularly discussed. The thus prepared alcohol-free hand sanitizer with its most innovative formulation, would potentially satisfy the public need for the green medical products.
Keywords: Antibacterial; Biomedical; Hand Sanitizer; Nanocellulose
Abbreviations: TPCN: Texas Poison Center Network; BAC: Benzalkonium Chloride; NFC: Nano Fibrillated Cellulose; GIB: Growth Inhibition of Bacteria; G-TPP: Gelatin- Encapsulated Tea Polyphenol; ORF-RE: Ontario Research Fund: Research Excellence; NSERC: Natural Sciences and Engineering Research Council; SEM: Scanning Electron Microscopy; TEM: Transmission Electron Microscope
Introduction
The spread of transmissible disease is considered as one of the main threats to public health [1]. The early symptoms of infection are sometimes non-specific and difficult to recognize, since most infectious diseases are caused by inadvertently contacting with pathogenic bacteria [2]. It therefore created a significant sanitation challenge to health care providers, and the effectiveness of hygiene primarily depends on the public compliance [3]. In general, handwashing is essential to remove the destructive bacterial from hands, thus lowering the threat of transmitting the disease to other individuals. The washing of hand can be implemented by either running water or waterless sanitizers [4]. Of which, handwashing with running water is considered as the most important and effective measure to prevent the transmission of infections. However, recent studies have shown that the compliance with water handwashing by healthcare workers is continuously decreasing to 20%-50% [5]. The deterrence to compliance includes the amount of time required for soap-and-water procedures with heavy workloads, skin irritation and dryness caused by frequently using of soap, and sometimes poor access to sinks [6]. Use of waterless hand sanitizers has thus been demonstrated as an alternative approach to overcome the above barriers to compliance. Hand sanitizers can be classified into two categories, alcohol-based and alcohol-free products. Of which, alcohol-based sanitizers typically contain 60%- 70% concentration of alcohol or other short-chained alcohols [7].
As an effective antibacterial agent, alcohol can immediately denature the target organisms, thus effectively killing most pathogens. However, alcohol is volatile and can cause severe dehydration of skins at its high concentration (>60%). The remained ingredients after the evaporation of alcohol from skin would have very limited antibacterial activity. Another issue is associated with the ingestion of alcohol-based sanitizer [8]. It is toxic and can cause serious poisoning, especially for young children. Miller et al. studied the exposures to alcohol-based hand sanitizer in Texas, US for the year of 2006 and 2007, based on the data from Texas Poison Center Network (TPCN). In 2006, there were 826 cases and 621 of them were ingestions occurred in children under 6 years of age; while the number increased to 737 of total 1022 cases in 2007 [9]. Furthermore, alcohol-based sanitizer is highly flammable and is forbidden in its large volume for airplane or public transportation. These data suggest that the use of alternative antibacterial actives might be a benefit in the medical/surgical setting. Alcohol-free formulations were thus been developed.
Alcohol-Free Sanitizer
Figure 1: SEM images of uncoated paper (a-1 (low magnification) & a-2 (high magnification)) and paper coated with 0.023 g TCSloaded NFC (b-1 (low magnification) & b-2 (high magnification)). Reprinted with permission from Carbohydrate Polymers.
Figure 2: Images of bacterial colonies with E. coli:
a) Paper without coating,
b) Paper coated with 0.023 g TCS-loaded NFC,
c) Paper coated with 0.33 g TCS-loaded NFC. Reprinted with permission from Carbohydrate Polymers.
Alcohol-free sanitizer is generally based on disinfectants/antibacterial agents and possesses both immediate and persistent effect. Viable antibacterial agents, such as benzalkonium chloride (BAC) or triclosan, can be used for this purpose and are active at relatively low concentrations (<0.5%) [10]. 72 Shown in (Figure 1) is a triclosan-based antibacterial formulation reported by Liu et al. [11].The triclosan (TCS) particles were loaded onto nano fibrillated cellulose (NFC) to prepare the antibacterial emulsion, which was then coated on the paper surface to test its antibacterial efficiency against Escherichia coli (E. coli, ATCC 11229). The results showed that 98.7% of the growth inhibition of bacteria (GIB) could be achieved at 0.023 g TCS-loaded NFC (Figure 2), which suggested a great potential of this triclosan-based emulsion for the medical use The alcohol-free sanitizer is mild to hand skin without disrupting its outer protection oil layer. The fragrance-free property is especially favorable for the users who are sensitive to the scent. Moreover, this type of hand sanitizer is financially superior over the alcohol-based products, especially for consumers who purchase in bulk such as schools or nosocomial offices.
Classification of Antimicrobial Agents
The selection of antimicrobial agents has to consider their antimicrobial activities, stability, side effects and cost [12]. The available antimicrobial agents for sanitizers can be classified into non-biobased and biobased agents. Of which, non-biobased agents, such as metal and their oxides, are widely used due to their unique sterilization activities towards wide variety of microbials [13]. They are usually present in the form of nanoparticles which can disrupt the unicellular membrane of microorganisms, thus leading to a decreased enzymatic activity. Besides metal oxides, Neosporin mupirocin and tetracycline can also be used as non-biobased antibacterial agents for the preparation of sanitizer [12]. As for the biobased antibacterial agents, a large group of low molecular weight compounds which are isolated from animals or plants, such as phenolics, terpenes, bacteriocins, peptides, fatty acids (lipids), organic acids as well as their compounds, can be used as biobased ingredients for antibacterial purpose [14]. Among them, Chitosan and polyphenols have been recognized as promising candidates of antimicrobial agents against a large spectrum of pathogens. Chitosan (CS) is a natural cationic polysaccharide with excellent biodegradability and nontoxicity [15]. It can be subjected to various chemical modifications due to its abundant surface hydroxyl and amino groups.
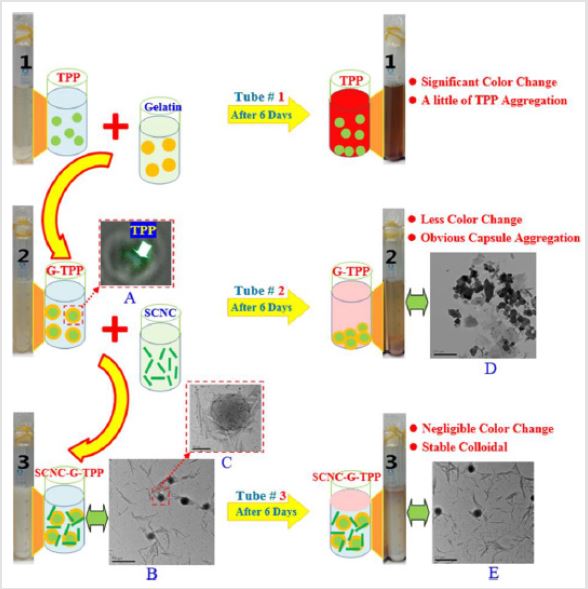
olyphenol, another type of representative antibacterial agent, has a wide range of applications such as pharmaceutical or food additives [16]. Particularly, polyphenol extracted from tea or fruits exhibits good water-solubility, which is especially suitable for the low-fat food and hydrophilic sanitizers. Despite its excellent performance, certain drawbacks still exist with this type of active oxidants, such as poor processing stability and undesired coloration during storage. A large number of studies has been conducted to address these issues, e.g. Sun et al. used sulfated nanocellulose (SCNC) as emulsifiers for stabilizing the gelatin-encapsulated tea polyphenol (G-TPP) and obtained well-entrapped SCNC-G-TPP capsules in their size of 92 ± 1nm (nanoscale) [17]. The residual TPP of SCNC-G-TPP was 85.7 ± 0.8% (HPLC Test) after a 6-day storage, which was much higher than that of G-TPP (44.9 ± 0.5%). The decrease of TPP after 40 min of storage at high temperature (121°C) was only 8.9 ± 0.3% (Free radical DPPH method) for SCNCG- TPP, which was distinct from that of G-TPP (17.2 ± 0.3%). Shown in (Figure 3) is the diagram which elucidates the function of SCNC in enhancing the strength of gelatin capsule through the hydrogen bonding between the hydroxyl groups of SCNC and amino groups of gelatins. The abundant sulfate groups of SCNC with high charge density, are capable to further stabilize the structure of SCNC-G-TPP capsules.
Figure 3: Understanding of different stabilities of TPP, G-TPP and SCNC-G-TPP.
A. Confocal Laser Microscope (CLSM) image at 70 nm by argon laser, showing the typical core–shell structure of G-TPP;
B. Transmission Electron Microscope (TEM) image of SCNC-G-TPP, showing the formation of SCNC-stabilized TPP
capsules (measuring bar of 0.5 μm);
C. TEM image of enlarged structures of SCNC-stabilized TPP gelatin capsule, showing that it was stable and intact
(measuring bar of 100 nm);
D. TEM image of G-TPP (after storage for 6 days), showing the capsule aggregates (measuring bar of 0.5 μm);
E. TEM image of SCNC-G-TPP (after storage for 6 days), showing the stable SCNC-stabilized TPP capsules (measuring bar
of 0.5 μm) (TPP concentration of 0.05 g/100 mL for all samples; initial pH of 4 for all test tubes; end pH of 3.9 for tube #1, 3.9
for tube #2 and 3.8 for tube #3, respectively). Reprinted with permission from Cellulose.
Biobased surfactants, which are derived from enzymatic fermentation, can also be used as antimicrobial agents for sanitizers. Different from the petroleum-based surfactants, the biobased products are with excellent biodegradability and negligible toxicity to their users. As for the antibacterial performance, Ren et al. investigated the synthesis of biobased surfactants and evaluated their inhibition of Escherichia coli O157:H7, Listeria monocytogenes and Salmonella Enteritidis [18]. Lipase was used for the preparation of glucose-fatty acid ester and the resulted glucose laurate (one kind of glucose ester) showed a significant growth inhibition of all the bacterial during 24 h.
Innovative Nanocellulose-Based Sanitizer
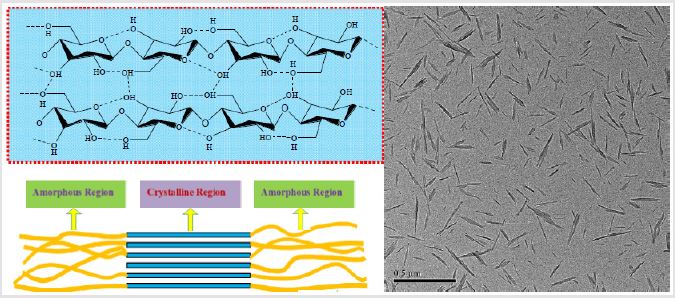
As for the selection of continuous phase for sanitizer, celluloseformed colloid can be considered as one of the candidates since it possesses ideal biocompatibility and excellent rheology properties. Cellulose, the most abundant biopolymer, consists of molecular chains of anhydro-D-glucopyranose units (AGU) [19]. Each AGU has three hydroxyl groups at its end (Figure 4). Its solid state has both low order (amorphous) and high order (crystalline) regions in the molecular structure. Cellulose can be disintegrated into nano-sized particles (nanocellulose) through chemical, mechanical/ enzymatic treatments or their combinations [20- 22]. Shown in (Figure 4) is the transmission electron microscope (TEM) image of the rod-like nanocellulose prepared by Sun et al., with length of 50-165 nm and width of 3-10 nm [23]. These nano-sized cellulose particles are readily to be dispersed into the aqueous solvents, due to the presence of intra- and inter- molecular hydrogen bonds. A stable colloid system can be formed due to the negative charges derived from the introduced sulfate groups on the surface of nanocellulose.
Figure 4: Molecular structure of cellulose and TEM image for its derived nanocellulose. Reprinted with permission from Journal of Cellulose.
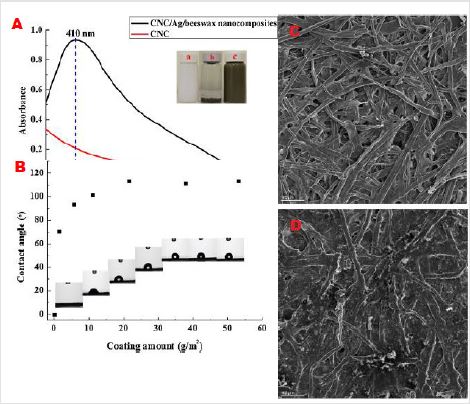
Figure 5: Characterization of the antimicrobial paper coated with nanocellulose/Ag/beeswax compounds.
A. UV-vis absorption spectra of the nanocellulose and nanocellulose/Ag/beeswax compounds (insert pictures of the
nanocellulose
a) silver nanoparticles and
b) nanocellulose/Ag/beeswax composites (c));
B. Contact angle of blank paper and paper coated with different amounts of nanocellulose/Ag/beeswax composites;
C. SEM images of the blank paper;
D. Paper coated with coating amount of 21.53 g/m2. Reprinted with permission from Carbohydrate Polymers.
In addition, nanocellulose sourced from biomass, and its use would generate a great environmental advantage compared with other non-biobased materials. These favorable attributes suggest that it can be potentially used for preparing the cellulose-based cream products, such as antibacterial gel. Liu et al. synthesized silver nanoparticles by reducing AgNO3 with sodium borohydride in the nanocellulose gel-medium [24]. The beeswax was then added into the above medium to prepare nanocellulose/Ag/beeswax antimicrobial gel for coating the paper. The immobilization of silver nanoparticles onto the antibacterial gel was confirmed by the UVvis spectra, which exhibited a typical silver absorption band at 410 nm (Figure 5a). Scanning electron microscopy (SEM) images of the coated paper showed a layered structure of the nanocellulose/Ag/ beeswax coating film (Figure 5c,d), which was responsible for the enhanced antibacterial activity against Escherichia coli (ATCC 11229). Furthermore, the coated paper had an improved water resistance as confirmed by the contact angle test (contact angle was 113.06° at the coating amount of 21.53 g/m2) (Figure 5b).
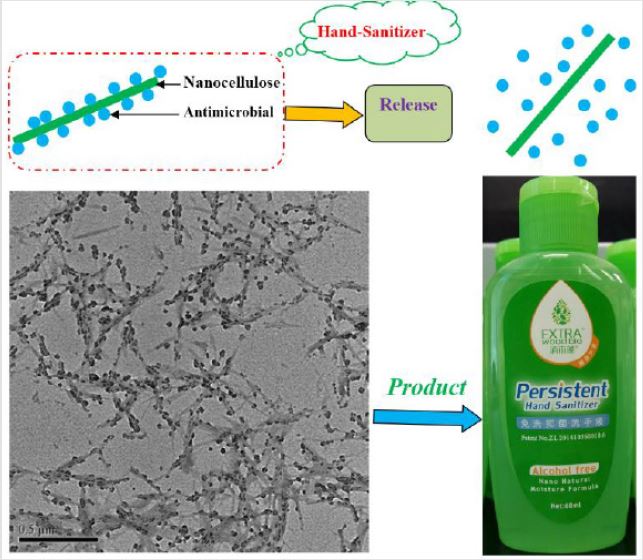
As a representative scale-up of nanocellulose-based antibacterial gel, EXTEA Woodbio hand sanitizer has been recently commercialized in China (product link: http://www.hxcellulose. com). Shown in (Figure 6) is the schematic which illustrates the homogenous loading of bio antimicrobials on the surface of nanocellulose particles (similar principle was previously reported by Sun et al. [25-27]). Government inspection was applied to detect the antibacterial potential of the sanitizer gel against Salmonella spp., Staphylococcus aureus and E. coli. The inhibition of bacterial growth of EXTRA Woodbio was compared with both blank samples (without adding of sanitizer) and control samples (two other commercial brands: one is alcohol- based and the other is alcohol-free product). The results showed a high instant antibacterial efficiency of more than 99.99% and 3 h persistent period of validity for EXTRA Woodbin, which were similar or superior to the commercial samples. Moreover, upon applying onto the skin, the thin layer of nanocellulose-film can lock the nutritional oil and further protect the skin from excessive dehydration. This unique bio affinity made the product most popular among different ages, especially for young children or female users.
Conclusion
he use of hand sanitizer as a supplement to water-washing provides an easy and efficient means of reducing the risk of cross-infection via body contact in the public area. Though the use of alcohol-based hand sanitizer is routine, it is now witnessing a continuous input for the developing of new alcohol-free sanitizer products. In particular, nanocellulose, an emerging biomaterial, is of great importance for the medical use due to its unique rheological properties. The innovative alcohol-free hand sanitizer formulated with nanocellulose, affords long-lasting antibacterial effect without skin dehydration, suits the public demand for green and healthy lifestyle.
Acknowledgment
The authors would like to acknowledge the financial support provided by National Natural Science Foundation of China (31501440), Hebei Provincial Scientific and Technological Cooperation & Development Foundation between Province and University of 2018, Tianjin Science and Technology Commissioner Program (16JCTPJC45300), Tianjin International Training Program for Excellent Postdoctoral Fellows of 2015, China Postdoctoral Science Foundation (2015M571268), Ford Motor Company (Canada), Total, Crest Mold Technology Inc., Stratus Plastics, Hawk Plastics, Topnotch plastics, Natural Sciences and Engineering Research Council (NSERC) and Ontario Research Fund: Research Excellence (ORF-RE).
Conflicts of Interest
The authors declare no conflict of interest in any form.
References
- Master D, Hess SL, Dickson H (1997) Scheduled hand washing in an elementary school population. Family medicine 29(5): 336-339.
- Yaun EA, Vasquez BA (2017) Antibacterial activity of formulated Psidium guajava (guava) hand sanitizer gel on Staphylococcus aureus. University of the Visayas-Journal of Research 11(1): 1-6.
- Alzyood M, Jackson D, Brooke J, Aveyard H (2018) An integrative review exploring the perceptions of patients and healthcare professionals towards patient involvement in promoting hand hygiene compliance in the hospital setting. Journal of clinical nursing 27(7-8): 1329-1345.
- Foddai AC, Grant IR, Dean M (2016) Efficacy of instant hand sanitizers against foodborne pathogens compared with hand washing with soap and water in food preparation settings: A systematic review. Journal of food protection 79(6): 1040-1054.
- Fendler EJ, Ali Y, Hammond BS, Lyons MK, Kelley MB, et al. (2002) The impact of alcohol hand sanitizer uses on infection rates in an extended care facility. American journal of infection control 30(4): 226-233.
- Boyce JM, Kelliher S, Vallande N (2000) Skin irritation and dryness associated with two hand-hygiene regimens: soap-and-water hand washing versus hand antisepsis with an alcoholic hand gel. Infection Control & Hospital Epidemiology 21(7): 442-448.
- Bondurant SW, Duley CM, Harbell JW (2019) Demonstrating the persistent antibacterial efficacy of a hand sanitizer containing benzalkonium chloride on human skin at 1, 2 and 4 hours after application. American journal of infection control 47(8): 928-932.
- Sun B, Zhang M, He Z, Zheng L, Shen J, et al. (2017) Towards greener and more sustainable cellulose-based hand sanitizer products. Journal of Bioresources and Bioproducts 2(2): 56-60.
- Miller M, Borys D, Morgan D (2009) Alcohol based hand sanitizers and unintended pediatric exposures: a retrospective review. Clinical pediatrics 48(4): 429-431.
- Dyer DL, Gerenratch KB, Wadhams PS (1998) Testing a new alcohol free hand sanitizer to combat infection. AORN journal 68(2): 239-251.
- Liu K, Chen L, Huang L, Ni Y, Sun B, et al. (2015) Enhancing antibacterial and strength of cellulosic paper by coating triclosan-loaded nano fibrillated cellulose (NFC). Carbohydrate polymers 117: 996-1001.
- Sun B, Kong F, Zhang M, Wang W, Birat Singh KC, et al. (2019) Cellulose-based antimicrobial composites and applications: a brief review. Paper and Biomass 4(4): 1-14.
- Hoseinnejad M, Jafari SM, Katouzian I (2018) Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications. Critical reviews in microbiology 44(2): 161-181.
- Fan X, Ngo H, Wu C (2018) Natural and bio-based antimicrobials: a review. In Natural and Bio-Based Antimicrobials for Food Applications. American Chemical Society 1-24.
- Dragostin OM, Samal SK, Dash M, Lupascu F, Pânzariu A, et al. (2016) New antimicrobial chitosan derivatives for wound dressing applications. Carbohydrate polymers 141: 28-40.
- Sun B, Wang W, He Z, Zhang M, Kong F, et al. (2018) Improvement of stability of tea polyphenols: A review. Current pharmaceutical design 24(29): 3410-3423.
- Sun B, Zhang M, Ni Y (2018) Use of sulfated cellulose nanocrystals towards stability enhancement of gelatin-encapsulated tea polyphenols. Cellulose 25(9): 5157-5173.
- Ren K (2018) Synthesis of some biobased surfactants, and their functionalities as emulsifiers and antimicrobial agents.
- Sun B, Zhang M, Shen J, He Z, Fatehi P, et al. (2019) Applications of cellulose-based materials in sustained drug delivery systems. Current medicinal chemistry 24: 1-17.
- Sun B, Hou Q, Liu Z, He Z, Ni Y, et al. (2014) Stability and efficiency improvement of ASA in internal sizing of cellulosic paper by using cationic ally modified cellulose nanocrystals. Cellulose 21(4): 2879-2887.
- Sun B, Hou Q, Liu Z, Ni Y (2015) Sodium periodate oxidation of cellulose nanocrystal and its application as a paper wet strength additive. Cellulose 22(2): 1135-1146.
- Sun B, Zhang M, Hou Q, Liu R, Wu T, et al. (2016) Further characterization of cellulose nanocrystal (CNC) preparation from sulfuric acid hydrolysis of cotton fibers. Cellulose 23(1): 439-450.
- Sun B, Wang W, Zhang M, Sain M (2018) Biomass based edible film with enhanced mass barrier capacity and gas permeable selectivity. Cellulose 25(10): 5919-5937.
- Liu K, Liang H, Nasrallah J, Chen L, Huang L, et al. (2016) Preparation of the CNC/Ag/beeswax composites for enhancing antibacterial and water resistance properties of paper. Carbohydrate polymers 142: 183-188.
- Sun B, Hou Q, He Z, Liu Z, Ni Y, et al. (2014) Cellulose nanocrystals (CNC) as carriers for a spiroxamine dye and its effect on photochromic efficiency. Carbohydrate polymers 111: 419- 424.
- Sun B, He Z, Hou Q, Liu Z, Cha R, et al. (2013) Interaction of a spiroxamine dye with latex and its photochromic efficiency on cellulosic paper. Carbohydrate polymers 95(1): 598-605.
- Sun B, Wang W, He Z, Zhang M, Kong F, et al. (2019) Biopolymer substrates in buccal drug delivery: current status and future trend. Current medicinal chemistry 25:1.

 Mini Review
Mini Review