Abstract
Aims: Erectile and endothelial dysfunctions are the most common complications
of diabetes. We studied the endothelial function and response to treatment of a topical
alprostadil in diabetics with erectile dysfunction.
Sample and Methods: 30 patients were enrolled in the non-intervention, postregistration,
prospective study with 300 μg alprostadil cream. We examined vascular
endothelial function using an ENDO-PAT 2000 device. We used International Index
of Erectile Function (IIEF-5), Erectile Hardness Score (EHS), Global Assessment
Questionnaire (GAQ) and Sexual Encounter Profile Q 2 and 3 (SEP 2, SEP 3) to assess
erectile function. The primary outcome measures were the proportion of patients with
an optimal treatment response at baseline and at 6 and 12 weeks after treatment and the
evaluation of patients and their partner’s subjective satisfaction.
Results: We diagnosed endothelial dysfunction in 15 diabetics. After 12 weeks of
treatment with alprostadil 53.3% of the sample, reached normal erectile function. We
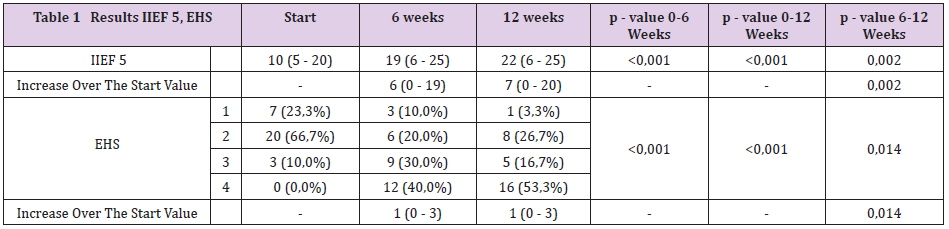
found a significant increase in IIEF-5 scores with a median of 6 points (range 0–19,
p<0.001) after 6 weeks compared to baseline, and of 7 points (range 0–20, p<0.001)
after 12 weeks of treatment compared to baseline; a significant increase occurred both
after 6 and 12 weeks of treatment (p=0.002). After both 6 and 12 weeks we found a
significant increase in erection rigidity, EHS, compared to baseline (p< 0.001, p = 0.014).
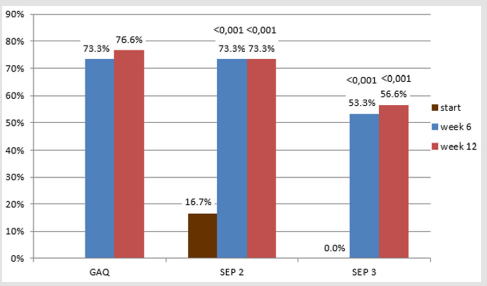
After 12 weeks of treatment, GAQ, improving erectile function was reached in 76.7%
patients.
Conclusion: We have demonstrated that topical alprostadil is efficient in diabetic
men with erectile-endothelial dysfunction.
Brief Summary
Erectile and endothelial dysfunctions are the most common complications of diabetes, we studied the endothelial function and response to treatment of a topical alprostadil in diabetics. We have demonstrated that topical alprostadil is efficient in diabetics with erectile-endothelial dysfunction.
Keywords: Erectile Dysfunction; Diabetes Type 2; Endothelial Dysfunction; Topical Alprostadil; International Index of Erectile Function; Erectile Hardness Score
Abbreviations: GAQ: Global Assessment Questionnaire; IIEF-5: International Index of Erectile Function; EHS: Erectile Hardness Score; SEP: Sexual Encounter Profile; ED: Erectile Dysfunction; PAD: Peripheral Arterial Disease; ABI: Ankle Brachial Index; PGE1: Prostaglandin E1; TSH: Thyroid- Stimulating Hormone; EF: Erectile Function; PRL: Prolactin
Introduction
Erectile dysfunction (ED) is the most common sexual complication of diabetes, affecting 35–75% of patients. Vascular disease as a hypertension, peripheral neuropathy, low level of testosterone and obesity are all more common in diabetics and predispose to developing ED. ED in diabetic patients is associated with endothelial dysfunction, atherosclerosis and coronary artery disease [1]. A key role in the pathogenesis of ED in diabetics is played by one’s decreased ability for corporal smooth muscle relaxation and an insufficient nitric oxide synthases system [2]. Cardiovascular disease leads to erectile dysfunction and increases the risk of mortality in diabetics. In our study we examined vascular endothelial function to diagnose endothelial-erectile dysfunction in diabetics. Ankle brachial index (ABI) is the principal screening method for peripheral arterial disease (PAD) in diabetics [3]. In our study, we measured vascular endothelial function with prediction: endothelial dysfunction is the first clinical stage of atherosclerosis. An ideal ED treatment in diabetics should be non-invasive, easily administered, effective, without serious adverse events, and without interaction with drugs, alcohol and food [1]. Alprostadil cream (Vitaros®, Herbacos Recordati) is a single-use topical cream approved for treatment of erectile dysfunction.
The active ingredient is alprostadil 300 μg, the pharmaceutical equivalent of endogenous prostaglandin E1 (PGE1). Alprostadil acts independently of the psychological and neurological components of the erectile function. Alprostadil is applied in drops to meatus of glans penis, where cream is absorbed directly into the corpora cavernosa proprietary skin permeation-enhancing delivery technology [4,5]. Topical alprostadil is recommended for men with vascular ED, who cannot tolerate or are not satisfied with oral therapy (sildenafil, tadalafil, vardenafil, avanafil) [6].
Subjects and Methods
This non-intervention, post-registration, prospective study with 300 μg topical alprostadil cream was approved by the State Institute for Drug Control of the Czech Republic, number of the study 1701250000. The study was conducted in accordance with the ethical principle of the Declaration of Helsinki. The study included 30 patients with type 2 diabetes, aged > 40 years, with mild to severe ED lasting for at least 6 months, who provided written consent to their participation in the study and who had a stable partner with regular sexual activity twice a week. The median age of diabetic patients was 55 years, ranging from 40 to 73 years. The median duration of type 2 diabetes was 10 years, ranging from 1 to 40 years. The median ED duration was 48 months, ranging from 6 to 216 months.
Methods
We examined vascular endothelial function using an ENDO-PAT 2000 device. The examination of the endothelium function was performed in the morning between 8 -10 am. Monitored variables included age, duration of type 2 diabetes and duration of ED, BMI (body mass index, obesity ≥30)), waist circumference (obesity ≥102 cm), HbA1c (glycated hemoglobin), lipid profile, glycaemia, total testosterone (tT, cutt-off: 12.1 nmol/l based on the guidelines of the European Urological Association), thyroid-stimulating hormone (TSH), and prolactin (PRL). Blood samples were taken between 8-10 a.m. The primary outcome measures was the proportion of patients with an optimal treatment response (IIEF-5≥22); other endpoints were mean change of IIEF-5, EHS the Sexual Encounter Profile Q2 and 3, the Global Assessment Question scores at baseline and at 6 and 12 weeks after treatment and the evaluation of patients and their partner’s subjective satisfaction. To assess erectile function (EF) before the start of treatment we used the validated, self-administered questionnaires for International Index of Erectile Function (IIEF-5), Erectile Hardness Score (EHS) and Sexual Encounter Profile Q 2 and 3 (SEP 2, SEP 3).
To analyze the effects of treatment, we used IIEF-5, EHS, the Global Assessment Questionnaire (GAQ, improvement of erection after treatment), SEP 2 (Were you able to insert your penis into your partner’s vagina?) and SEP 3 (Did your erection last long enough for you to have successful intercourse?) [7] ED severity was classified into 4 categories based on IIEF-5 scores: ≤6 - severe ED, 8–16 – moderate, 17–21 - mild, and 22–25 - none [8]. Erectile Hardness Score is based on self-estimated rigidity, categorized by degrees 1–4: 1 and 2 being insufficient for insertion into the vagina, 3 and 4 being sufficient for sexual intercourse. At degree 0, the penis does not enlarge, at degree 1 the penis is larger but not hard, at degree 2 the penis is hard but not hard enough for penetration, at degree 3 the penis is hard enough for penetration but not completely hard, at degree 4 the penis is completely hard and fully rigid for coitus [9].
Study design
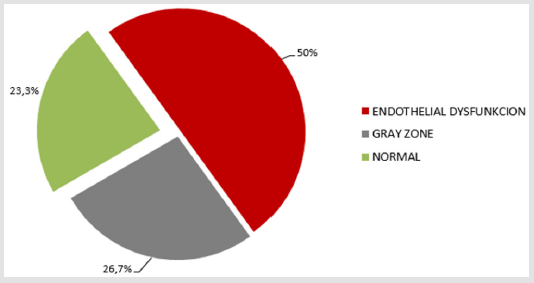
The study consisted of a 4-week, treatment-free run-in period, followed by a 12-week treatment phase. The screening visit after the run-in period included collecting the patient’s detailed medical and sexual history, a somatosexual examination, basic cardiologic and neurologic status and a laboratory examination. Men with diabetes were stratified by ED severity according to IIEF-5, EHS and SEP 2. Sonographic examination of the penis was performed in order to rule out Peyronie’s disease. We examined endothelial function using a non-invasive ENDO-PAT 2000 device. It assesses endothelium-mediated change in the vascular tonus caused by a 5-minutes occlusion of the brachial artery in the non-dominant arm. After the occlusion has been released, a pulse wave causes an endothelium-mediated vasodilatation. The dilatation is manifested as a reactive hyperemia and the ENDO-PAT device reacts by increasing the signal amplitude. The ratio of PAT signal pre-occlusion and post-occlusion assessment is carried out automatically by ENDO-PAT software. The resulting RHI (Reactive Hyperemia Index) is >2.07 for a healthy person, less than 1.67 for endothelial dysfunction, and 1.67–2.07 is considered a “grey zone” [10].
The patient was thoroughly instructed on how to use the alprostadil cream, followed by a demonstration and training of the application on a model, were instructed to only use topical alprostadil before coital activity at a frequency of twice per week, with 24 doses in total. Treatment efficacy, adverse events and treatment satisfaction were assessed at 6 and 12 weeks. We monitored adverse events in both the patient and his partner. We assessed subjective satisfaction with topical alprostadil ED treatment on a three-degree scale: very satisfied – 1, somewhat satisfied – 2, not satisfied – 3. The female partner’s satisfaction with treatment was assessed on a three-degree scale: high – 1, moderate - 2, low – 3.
Statistics
Standard descriptive statistics were applied in the analysis; absolute and relative frequencies for categorical variables and median supplement with min-max range for continuous variables. The statistical significance of differences between groups of patients was tested using the Fisher exact test for categorical variables and the Mann-Whitney test for continuous variables; statistical significance of differences between time points was tested using the McNemar test for categorical variables and the Wilcoxon paired test for continuous variables. Statistical analysis was computed using SPSS 25.0.0.1.
Results
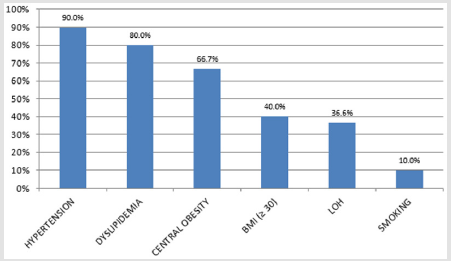
We managed to collect data for all 30 patients. All patient was highly motivated, none of the patients ended treatment prematurely. TSH and PRL hormones were normal in all patients. We confirmed late-onset hypogonadism in 11 patients (36.7%); testosterone replacement therapy was administered before starting alprostadil treatment. We found a high incidence of risk factors (Figure 1). Median HbA1c was 55 mmol/mol (7.2 %). Endothelial dysfunction, RHI <1.67, was found in 15 diabetics, 50 % (Figure 2). After 12 weeks of treatment, normal erectile function (EF>22) was found in 16 patients (53.3%). We found a significant increase in IIEF-5 after 6 and 12 weeks compared to baseline; a significant increase was found between 6 and 12 weeks of treatment. After 6 weeks, we found a significant EHS change compared to baseline, rigidity also increased in week 12 (Table 1). 76.7% of the sample described improved erections (GAQ) after 12 weeks of treatment. After 6 and 12 weeks, we found a significant SEP 2 and SEP 3 change compared to baseline (Figure 3). After 12 weeks of treatment, 50 % of the diabetics were very satisfied, and 50 % their partners reported high satisfaction with the treatment.
We found the correlation between treatment effect and variables. IIEF-5 scores more 22 (normal erectile function) after 6 weeks of treatment were reached significantly more often in patients with higher IIEF-5 or EHS at baseline (p=0.003 and p=0.048, respectively) who were not obese (p=0.018); after 6 and 12 weeks of patients with lower triglyceride levels (p=0.027 and 0.020, respectively). Patients who reached better erection rigidity as measured by EHS had statistically significantly lower level of triglycerides (p=0.037). Satisfaction with treatment correlated with higher IIEF-5 score at baseline compared to week 6 and 12, (p=0.042 and 0.047, respectively), and lower triglycerides levels at week 6 and at week 12 (p=0.014 and p=0.044, respectively). The most common adverse event was penile burning at week 6 and 12, reported by 7 patients (23.3%). Penile pain occurred in 6 patients (20%) at week 6, and in 1 patient (3.3%) at week 12. In 2 cases (6.7%), female partners described vaginal pain at week 6, and 1 partner (3.3%) at week 12.
Discussion
Sexual dysfunction can have a major impact on quality of life and psychosocial and emotional well-being [11]. ED is a common comorbidity and usually an organic complication [1]. The prevalence of ED is greater in diabetic patients and, likewise, men with diabetes are at a significantly higher risk of ED [12]. We found that topical treatment with alprostadil caused a significant improvement in erectile function. After 12 weeks of treatment, more than half of the patients reached a normal erectile function but improving of erection described 76.7 % of patients. Better therapeutic response to treatment with topical alrostadil had diabetics with better control of diabetes - without obesity, with normal level of triglycerides and better erectile function at baseline. The diabetics represent a group of ED patients that is difficult to treat. Men with diabetes are available to cardiovascular and neurological complications that can often result in a higher incidence of ED compared with general population [1]. But ED in diabetic patients requires appropriate attention, as it may be the first manifestation of endothelial dysfunction, or even systemic atherosclerosis including coronary artery disease.
In half of the sample we found endothelial dysfunction. These patients should be carefully monitored for the development of atherosclerosis. According to Guidelines European Association of Urology oral treatment of ED is the first line of therapy [13]. Although PDE5i (phosphodiesterase 5 inhibitors), as a first line therapy are effective, are associated with treatment failure in up to half of patients [14]. Phosphodiesterase 5 inhibitors are less effective in diabetics compared to the general population [12]. The reason for the low efficacy of PDE5i is nerve-vascular severe damage in diabetics. Efficacy of PDE5i is lower in diabetics to impaired endothelium-derived factors in penile arteries and underlying endothelial dysfunction [12]. Endothelial dysfunction was found in 50 % diabetics in our study. Topical treatment is indicated for men with a vascular ED, the vasodilating effect of alprostadil cream on penis hemodynamics is especially beneficial for diabetic patients with endothelial dysfunction, hence the endothelial dysfunction finding did not predict the treatment effect, as confirmed in our study. A number of studies have monitored the effect of PDE5i in diabetics, which is lower than in the general population.
After 12 weeks of treatment with sildenafil, 56% of 131 diabetic patients reported improved erections compared to 10% of 127 patients taking a placebo [15]. In a study of 216 patients with diabetes and ED, 56% of patients receiving 10 mg tadalafil and 64% receiving tadalafil 20 mg had significantly improved erections compared with those taking placebo [16]. In our study with topical alprostadil, we demonstrated improved erections in 76.6%. In contrast to PDE5i, alprostadil cream circumvents the barrier of non-functional nerves of diabetic patients with neuropathy and acts directly in erectile tissue . Alprostadil acts independently of the psychological factors and neurological components of the entire process that lead to erection [17]. For a long time, alprostadil is available as an intracavernous injection. Intracavernosal therapy is also often effective in ED refractory to treatment with PDE5i, especially in diabetics. But intracavernosal therapy is an invasive procedure that is associated with dropout rates as high as 40–50% due to pain, penile fibrosis, hematoma and fear of needles too [18- 20]. Alprostadil cream combines the efficacy of alprostadil with an easy-to-use formulation (compared to auto injection therapy). In contrast to PDE5i, which require an erectogenic stimulus to activate the nitric oxide/guanylate cyclase pathway, Alprostadil cream acts directly in erectile tissue.
Patients who may benefit are difficult-to-treat patients with diabetes or cardiovascular disease and patients after radical prostatectomy [17]. 83% of patients with severe ED receiving 300 μg topical alprostadil reported significant improvement of erection compared with 26% taking placebo, 49% had diabetes, p< 0.001 [17]. Padma–Nathan et al. presented results of two multicenter, placebo-controlled phase 2 studies. The percentage of patients reporting an improvement in erection was significantly greater in the active drug group (p<0.001): 93% of the 0.2 mg treatment group in the first study, and 83% of the 0.3 mg treatment group in the second study [21]. Topical alprostadil cream was safe and well tolerated, with most adverse events localized to site of application, and included penile burning, genital pain, and genital erythema [22]. All local adverse events were mild or moderate and of short duration. Topical alprostadil in this study was well tolerated with the most common local adverse event being urogenital pain; the vast majority of these adverse events (<97%) were of mild or moderate intensity and short duration [21]. With continued treatment, the adverse events decreased in both the patients and their female partners [23], as confirmed in our study.
The most common adverse event in our study was burning sensation in the penis; no patient withdrew from the study because of adverse events. Because of the prostaglandin content in semen, exposure to the active ingredient of topical alprostadil represents a low risk for female partners [3]. Better health, milder ED degree, lower level of triglycerides predicted a better treatment outcome in our study. Triglycerides are related to insulin resistance. It is safe to deduce that lower levels of triglycerides lead to less serious disorders and easier treatment. In the future is important to examine the efficacy and safety of topical alprostadil in a general ED population, investigating the long-term outcomes, and studying the efficacy and safety in difficult-to-treat patients, such as men with prostate carcinoma, after radical prostatectomy: topical alprostadil (Vitaros®) was administered in seventy-four patients underwent non-nerve sparing robot assisted radical prostatectomy(RARP) ≥ twice a week. IIEF-5, EHS, SEP 2, 3 and GAQ were analyzed. Six patients dropped out, 89.7% showed a positive SEP 2, 77.8% SEP 3, 17.6% switched to intracavernous injection therapy. The authors concluded, Vitaros may become a viable alternative to common injection therapies in patients after RARP [24]. Topical alprostadil may be recommended for patients with less curable ED as an alternative to intracavernous injection therapy.
Conclusion
Erectile and endothelial dysfunctions are the most common complications of diabetes. We studied the endothelial function and response to treatment of a topical alprostadil in diabetics suffer from ED. Endothelial dysfunction was found in 50 % diabetics in our study. The vasodilating efficacy of alprostadil cream on penis hemodynamics is especially beneficial for diabetic patients with endothelial dysfunction, hence the endothelial dysfunction finding did not predict the treatment effect, as confirmed in our study. We found a significant improving erectile function in 76.7 % of respondents, significant increase in IIEF-5 and EHS scores compared to baseline. After 12 weeks of treatment, half of diabetics were very satisfied, and half of their partners reported high satisfaction with the treatment. Better therapeutic response to treatment with topical alrostadil had diabetics with better control of diabetes - without obesity, with normal level of triglycerides and better erectile function at baseline. We conclude that topical alprostadil is a challenge not only for andrologists, but also for diabetologists and GP who want to help improve the sex life of diabetics who do not respond to PDE5i.
Search Strategy and Selection Criteria
Erectile and endothelial dysfunctions are the most common complications of diabetes. We studied the endothelial function and response to treatment of a topical alprostadil in diabetics. The study included 30 patients with type 2 diabetes, aged > 40 years, with mild to severe ED lasting for at least 6 months, who provided written consent to their participation in the study and who had a stable partner with regular sexual activity twice a week. This non-intervention, post-registration, prospective study with 300 μg topical alprostadil cream was approved by the State Institute for Drug Control of the Czech Republic, number of the study 1701250000.
Competing Interests’ Statement
The authors declare no conflict of interest in relation to the work described.
Funding
None
Acknowledgments
We would like to thank Mediol for the use of the ENDO-PAT 2000 device for our study, and University Hospital Brno, Czech Republic.
Author Contributions
1. Tatjana Sramkova carried out the conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript and revised it for intellectual content.
2. Jiri Jarkovsky carried out the statistical analysis, interpretation of data, tables and figures and helped to draft the manuscript and revised it for intellectual content.
3. Katerina Sramkova carried out the acquisition of data, analysis of data and helped to draft the manuscript.
4. Michal Pohanka helped tu draft the manuscript.
5. Ales Cermak helped tu draft the manuscript.
6. All authors read and approved the final manuscript.
References
- Cummings MF (2004) The impact of erectile dysfunction and its treatment with phosphodiesterase type-5 (PDE5) inhibitors in patients with diabetes. Pract Diab Int 21(6): 225-230.
- Fonseca V, Seftel A, Denne J, Fredlund P (2004) Impact of diabetes mellitus on the severity of erectile dysfunction and response to treatment: analysis of data from tadalafil clinical trials. Diabetologia 47: 1914-1923.
- Homza M, Machaczka O, Porzer M, Kozak M, Plasek J, et al. (2019) Comparison of different method of ABI acquisition for detection of peripheral artery disease in diabetic patients. Biomed Pap 163(3): 227-232.
- Meier Davis SR, Debar S, Siddoway J, Rabe M (2015) Daily application of alprostadil topical cream (Vitaros) does not impact vaginal pH, flora, or histology in female cynomolgus monkeys. International Journal of Toxicology 34 (1): 11-15.
- Moncada I, Cuzin B (2015) Clinical efficacy and safety of Vitaros/Virirec (Alprostadil cream) for the treatment of erectile dysfunction. Urologia 82(2): 84-92.
- Anaissie J, Hellstrom WJG (2016) Clinical use of alprostadil topical cream in patients with erectile dysfunction: A review. Research and Reports in Urology 8: 123-131.
- Araujo AB, Allen KR, NI X, Rosen RC (2012) Minimal clinically important differences in the vaginal insertion and successful intercourse items of the sexual encounter profile. J Sex Med 9(1): 169-179.
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, et al. (1997) The International Index of Erectile Function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology 49(6): 822-830.
- Goldstein I, Mulhall P, Buskmain AG, Cappellei JC, Hoidstein K, et al. (2008) The erection hardness score and its relationship to successful sexual intercourse. J Sex Med 5(10): 2374-2380.
- Axtell AL, Gomari FA, Cooke JP (2010) Assessing endothelial vasodilator function with the Endo-PAT 2000. J Vis Exp 15 (44): 1-5.
- Montorsi F, Adaikan G, Becher F, Giuliano F, Khoury S, et al. (2010) Summary of the recommendations on sexual dysfunction in men. J Sex Med 7(11): 3572-3588.
- Hatzichristou D, Gambla M, Rubio-Auriolest E, Buvat J, Brocks GB, Spera G, et al. (2008) Efficacy of tadalafil once daily in men with diabetes mellitus and erectile dysfunction. Diabetic Medicine 25 (2): 138-146.
- Hatzimouratidis K, Giuliano F, Moncada I, Muneer A, Salonia A, et al. EAU Guidelines on male sexual dysfunction: Erectile dysfunction and premature ejaculation.
- Calvaiheira AA, Pereira NM, Maroco J, Forjaz V (2012) Dropout in the treatment of erectile dysfunction with PDE5: a study on predictors and a qualitative analysis or reasons for discontinuation. J Sex Med 9(9): 2361 -2369.
- Rendell MS, Rajfer J, Wicker PA, Smith MD (1999) Sildenafil for treatment of erectile dysfunction in men with diabetes: A randomized controlled trial. Sildenafil Diabetes Study Group. Jama 281(5): 421-426.
- Saenz de Tejada I, Anglin G, Knight JR, Emmick JT (2002) Effects of tadalafil on erectile dysfunction in men with diabetes. Diabetes Care 25(12): 2159-2164.
- Cuzin B (2016) Alprostadil cream in the treatment of erectile dysfunction: Clinical evidence and experience. The Adv Urol 8(4): 249-256.
- Linet OI, Ogrinc FG (1996) Efficacy and safety of intracavernosal alprostadil in men with erectile dysfunction. The Alprostadil study group. N Engl Med 334(14): 873-887.
- Kamenov ZA (2015) A comprehensive review of erectile dysfunction in men with diabetes. Exp Clin Endocrinol Diabetes 123(3): 141-158.
- Heaton JP, Lording D, Liu SN, Litonjua AD, Guangwei L, et al. (2001) Intracavernosal alprostadil is effective for the treatment of erectile dysfunction in diabetic men. Int J Impot Res 13(6): 317-321.
- Padma Nathan H, Steidle C, Salem S, Tayse N, Yeager J, et al. (2003) The efficacy and safety of a topical alprostadil cream, Alprox-TD for the treatment of erectile dysfunction: Two phase 2 studies in mild-to moderate and severe ED. Int J Impot Res 15(1): 10-17.
- Padma Nathan H, Yeager JL (2006) An integrated analysis of alprostadil topical cream for the treatment of erectile dysfunction in 1732 patients. Urology 68(2): 386-391.
- Mulhall J, Porst H, Goldstein I (2013) Comparison of Vitaros efficacy and safety with short- term and longer-term use. J Sex Med 10: 264-265.
- Della Camera PA, Morselli S, Cito G, Tasso G, Laruccia N, et al. (2018) Topical alprostadil (Vitaros®) in the treatment of erectile dysfunction after non-nerve-sparing radical prostatectomy. Urologia 85(2): 55-59.

 Research Article
Research Article